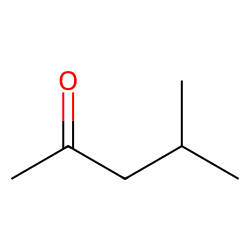
Physical Properties
Temperature Dependent Properties
Property
Value
Unit
Temperature (K)
Source
Cp,gas [180.50; 187.90]
J/mol×K
[394.30; 415.75]
Cp,gas 180.50 ± 0.27
J/mol×K
394.30
NIST
Cp,gas 182.26 ± 0.27
J/mol×K
399.44
NIST
Cp,gas 184.31 ± 0.28
J/mol×K
405.15
NIST
Cp,gas 186.06 ± 0.28
J/mol×K
410.70
NIST
Cp,gas 187.90 ± 0.28
J/mol×K
415.75
NIST
Cp,liquid 211.90
J/mol×K
298.15
NIST
η [0.0002910; 0.0061867]
Pa×s
[192.31; 390.11]
η 0.0061867
Pa×s
192.31
Joback Calculated Property
η 0.0025603
Pa×s
225.28
Joback Calculated Property
η 0.0013273
Pa×s
258.24
Joback Calculated Property
η 0.0007984
Pa×s
291.21
Joback Calculated Property
η 0.0005326
Pa×s
324.18
Joback Calculated Property
η 0.0003828
Pa×s
357.14
Joback Calculated Property
η 0.0002910
Pa×s
390.11
Joback Calculated Property
Δvap H [34.49; 42.50]
kJ/mol
[308.00; 389.40]
Δvap H 40.10 ± 0.10
kJ/mol
308.00
NIST
Δvap H 39.00 ± 0.10
kJ/mol
323.00
NIST
Δvap H 38.00 ± 0.10
kJ/mol
338.00
NIST
Δvap H 42.50
kJ/mol
340.50
NIST
Δvap H 41.20
kJ/mol
342.00
NIST
Δvap H 37.60
kJ/mol
347.00
NIST
Δvap H 37.40 ± 0.10
kJ/mol
348.00
NIST
Δvap H 38.70
kJ/mol
359.00
NIST
Δvap H 39.20
kJ/mol
362.50
NIST
Δvap H 37.00
kJ/mol
369.00
NIST
Δvap H 40.61
kJ/mol
388.90
NIST
Δvap H 34.49
kJ/mol
389.40
NIST
Pvap [3.89; 114.12]
kPa
[303.15; 393.15]
Pvap 3.89
kPa
303.15
Density...
Pvap 5.88
kPa
313.15
Phase e...
Pvap 9.42
kPa
323.15
Phase e...
Pvap 13.85
kPa
331.98
Phase e...
Pvap 14.56
kPa
333.15
Phase e...
Pvap 19.35
kPa
340.18
Vapor L...
Pvap 19.78
kPa
340.66
Vapor L...
Pvap 20.72
kPa
341.81
Phase e...
Pvap 21.82
kPa
343.15
Phase e...
Pvap 21.92
kPa
343.42
Vapor L...
Pvap 30.01
kPa
351.55
Vapor L...
Pvap 30.29
kPa
351.81
Phase e...
Pvap 31.12
kPa
352.71
Vapor L...
Pvap 31.71
kPa
353.11
Vapor L...
Pvap 31.80
kPa
353.15
Phase e...
Pvap 39.35
kPa
359.22
Vapor L...
Pvap 39.63
kPa
359.39
Vapor L...
Pvap 40.05
kPa
359.58
Vapor L...
Pvap 43.51
kPa
362.04
Phase e...
Pvap 44.70
kPa
362.88
Vapor L...
Pvap 45.19
kPa
363.15
Phase e...
Pvap 49.68
kPa
365.90
Vapor L...
Pvap 50.66
kPa
366.61
Vapor L...
Pvap 53.01
kPa
368.06
Vapor L...
Pvap 53.42
kPa
368.07
Vapor L...
Pvap 53.26
kPa
368.14
Vapor L...
Pvap 53.24
kPa
368.15
Vapor L...
Pvap 54.29
kPa
368.70
Vapor L...
Pvap 59.43
kPa
371.34
Vapor L...
Pvap 59.75
kPa
371.64
Vapor L...
Pvap 60.19
kPa
371.91
Vapor L...
Pvap 60.56
kPa
372.03
Phase e...
Pvap 62.78
kPa
373.15
Phase e...
Pvap 65.18
kPa
374.37
Vapor L...
Pvap 69.16
kPa
376.26
Vapor L...
Pvap 69.98
kPa
376.49
Vapor L...
Pvap 74.83
kPa
378.80
Vapor L...
Pvap 79.56
kPa
380.65
Vapor L...
Pvap 80.16
kPa
381.06
Vapor L...
Pvap 82.62
kPa
382.04
Phase e...
Pvap 84.16
kPa
382.66
Vapor L...
Pvap 85.44
kPa
383.15
Phase e...
Pvap 90.58
kPa
384.99
Vapor L...
Pvap 98.70
kPa
388.07
Vapor L...
Pvap 99.40
kPa
388.31
Vapor L...
Pvap 100.35
kPa
388.63
Vapor L...
Pvap 100.41
kPa
388.65
Vapor L...
Pvap 101.85
kPa
389.06
Vapor L...
Pvap 101.30
kPa
389.15
Vapor-L...
Pvap 102.78
kPa
389.49
Vapor L...
Pvap 110.74
kPa
392.07
Phase e...
Pvap 114.12
kPa
393.15
Phase e...
n 0 [1.39327; 1.39586]
[293.15; 298.15]
n 0 1.39586
293.15
Vapor L...
n 0 1.39355
298.15
Isobari...
n 0 1.39360
298.15
Bubble ...
n 0 1.39350
298.15
Excess ...
n 0 1.39327
298.15
Ternary...
n 0 1.39420
298.15
Isobari...
n 0 1.39360
298.15
Liquid-...
ρl [773.00; 805.12]
kg/m3
[288.15; 323.15]
ρl 805.12
kg/m3
288.15
Thermal...
ρl 801.00
kg/m3
293.00
KDB
ρl 800.80
kg/m3
293.15
Evaluat...
ρl 800.90
kg/m3
293.15
Effect ...
ρl 800.00
kg/m3
293.15
Thermop...
ρl 801.00
kg/m3
293.15
Thermop...
ρl 799.61
kg/m3
293.20
Liquid-...
ρl 799.00
kg/m3
298.15
Liquid ...
ρl 798.09
kg/m3
298.15
Evaluat...
ρl 800.59
kg/m3
298.15
Liquid-...
ρl 795.98
kg/m3
298.15
Thermal...
ρl 795.94
kg/m3
298.15
Liquid ...
ρl 796.23
kg/m3
298.15
Liquid-...
ρl 796.19
kg/m3
298.15
Excess ...
ρl 799.80
kg/m3
298.15
Liquid ...
ρl 796.50
kg/m3
298.15
Isother...
ρl 796.03
kg/m3
298.15
Evaluat...
ρl 796.10
kg/m3
298.15
Evaluat...
ρl 792.00
kg/m3
303.15
Thermop...
ρl 791.25
kg/m3
303.15
Excess ...
ρl 791.60
kg/m3
303.15
Evaluat...
ρl 796.33
kg/m3
303.15
Excess ...
ρl 786.75
kg/m3
308.15
Thermal...
ρl 786.90
kg/m3
308.15
Evaluat...
ρl 782.30
kg/m3
313.15
Evaluat...
ρl 782.00
kg/m3
313.15
Thermop...
ρl 781.11
kg/m3
313.20
Liquid-...
ρl 777.60
kg/m3
318.15
Evaluat...
ρl 777.46
kg/m3
318.15
Thermal...
ρl 773.00
kg/m3
323.15
Evaluat...
csound,fluid [1138.00; 1175.00]
m/s
[303.15; 313.15]
csound,fluid 1175.00
m/s
303.15
Densiti...
csound,fluid 1156.00
m/s
308.15
Densiti...
csound,fluid 1138.00
m/s
313.15
Densiti...
Datasets
Viscosity, Pa*s
Fixed
Measured
Temperature, K - Liquid
Pressure, kPa - Liquid
Viscosity, Pa*s - Liquid
308.15
101.30
0.0005
Reference
Correlations
Similar Compounds
Find more compounds similar to Methyl Isobutyl Ketone .
Mixtures
Find more mixtures with Methyl Isobutyl Ketone .
Sources
KDB Pure (Korean Thermophysical Properties Databank) KDB Vapor Pressure Data Crippen Method Isothermal vapour liquid equilibria in the binary and ternary systems composed of 2-propanol, diisopropyl ether and 4-methyl-2-pentanone Liquid liquid equilibria for the quaternary system methyl isobutyl ketone water phenol hydroquinone Isobaric vapour liquid equilibria for the binary systems 4-methyl-2-pentanone + 1-butanol and + 2-butanol at 20 and 101.3 kPa Compact apparatus for rapid measurement of high-pressure phase equilibria of carbon dioxide expanded liquids Isothermal vapour-liquid equilibria in the binary and ternary systems composed of 2,2,4-trimethylpentane, 2-methyl-1-propanol, and 4-methyl-2-pentanone Selection of entrainers for the separation of the binary azeotropic system methanol + dimethyl carbonate by extractive distillation Measurements and correlation of liquid-liquid equilibria of 4-methyl-2-pentanone + ethanol + water and 4-methyl-2-pentanone + n-butanol + water ternary systems between 283.2 and 323.2 K Solubility of lansoprazole in different solvents Liquid-liquid equilibria for the pseudo-ternary system {aqueous sulfuric acid solution + methyl ethyl ketone or methyl isopropyl ketone + phosphonium-based ionic liquids} at 298.15 K and atmospheric pressure Liquid liquid equilibria of 4-methyl-2-pentanone + 1-propanol or 2-propanol + water ternary systems: Measurements and correlation at different temperatures Isothermal vapor-liquid equilibrium and excess molar enthalpies of the binary mixtures furfural + methyl isobutyl ketone, + 2-butanol and + 2-methyl-2-butanol Liquid-liquid equilibria of water + lactic acid + methyl isobutyl ketone Solubility and tie-line data for ternary aqueous mixtures of cyclopentanol with organic solvents at T = 298.2 K: Experiments and NRTL model Activity coefficients at infinite dilution for different alcohols and ketones in [EMpy][ESO4]: Experimental data and modeling with PC-SAFT Salt influence on MIBK/water liquid-liquid equilibrium: Measuring and modeling with ePC-SAFT and COSMO-RS Liquid-liquid equilibria for methyl isobutyl ketone + cresols + water at 333.15K, 343.15K and 353.15K: Experimental results and data correlation Liquid-liquid equilibrium for ternary systems, methyl isobutyl ketone +(catechol, resorcinol and hydroquinone) + water at 333.15 K, 343.15 K and 353.15 K Measurement of the solubility of the salt of 2- mercaptobenzothiazole with cyclohexylamine and tert-butylamine in various solvents at low temperatures: Models and thermodynamic parameters The solubility of maleic acid in various inorganic salts and organic solvents: Thermodynamics model and parameters Liquid-liquid equilibria of ternary and quaternary systems involving 5-hydroxymethylfurfural, water, organic solvents, and salts at 313.15 K and atmospheric pressure Liquid-liquid equilibrium data and thermophysical properties for ternary systems composed of water, acetic acid and different solvents Salt effect on liquid-liquid equilibria of tetrahydrofuran/water/5-hydroxymethylfurfural systems Salting-out effect of NaCl and KCl on the ternary LLE data for the systems of (water + propionic acid + isopropyl methyl ketone) and of (water + propionic acid + isobutyl methyl ketone) Thermodynamics of binary mixtures of N-methyl-2-pyrrolidinone and ketone. Experimental results and modelling of the solid-liquid equilibrium and vapou-liquid equilibrium. The Modified UNIFAC (Do) model characterization Bubble temperature measurements on seven binary mixtures formed by ethylbenzene at 94.7 kPa Density, viscosity, isothermal (vapour + liquid) equilibrium, excess molar volume, viscosity deviation, and their correlations for chloroform + methyl isobutyl ketone binary system Excess enthalpies and (vapour + liquid) equilibrium data for the binary mixtures of dimethylsulphoxide with ketones Excess molar volumes and ultrasonic studies of dimethylsulphoxide with ketones at T = 303.15 K Excess molar volumes and ultrasonic studies of N-methyl-2-pyrrolidone with ketones at T = 303.15 K Phase equilibrium properties of binary mixtures containing 2,5-dimethylfuran and furfuryl alcohol or methyl isobutyl ketone at several temperatures Thermophysical approach to understand the nature of molecular interactions and structural factor between methyl isobutyl ketone and organic solvents mixtures Effect of 1-ethyl-3-methylimidazolium tetrafluoroborate on the phase equilibria for systems containing 5-hydroxymethylfurfural, water, organic solvent in the absence and presence of sodium chloride Excess molar enthalpies of methyl isobutyl ketone (MIBK) with alkan-1-ols (C1-C6) and their correlations at 298.15 K Evaluation of thermodynamic properties of fluid mixtures by PC-SAFT model Ternary and Binary LLE Measurements for Solvent (4- Methyl-2-pentanone and 2-Methyl-2-butanol) + Furfural + Water between 298 and 401 K Solubility of Benzoic Acid and Aspirin in Pure Solvents Using Focused Beam Reflective Measurement Influence of Salt on the Solubility and Tie-Line Data for Water + Formic Acid + Methyl Isobutyl Ketone at T = 298.15 K Equilibrium Study on the Extraction of Levulinic Acid from Aqueous Solution with Aliquat 336 Dissolved in Different Diluents: Solvent s Polarity Effect and Column Design Liquid-Liquid Equilibria of Ionic Liquids-Water-Acetic Acid Mixtures Solubility Measurement and Correlation of Two Erlotinib Hydrochloride Polymorphs in Alcohols and Ketones from 273.15 to 323.15 K Evaluation of Diethyl Carbonate and Methyl Isobutyl Ketone as Entrainers for the Separation of 1-Hexene and n-Hexane Determination and Thermodynamic Modeling of Solid Liquid Phase Equilibrium for Esomeprazole Sodium in Monosolvents and in the (Ethanol + Ethyl Acetate) Binary Solvent Mixtures Vapor Liquid Equilibrium for Methyl Isobutyl Ketone (MIBK) + (1-Propanol or 2-Propanol) Binary Mixtures Thermal and Volumetric Properties of Some C5 and C6 Alkanones at Infinite Dilution in Water Measurement and Thermodynamic Modeling of Ternary (Liquid + Liquid) Equilibrium for Extraction of N,N-Dimethylacetamide from Aqueous Solution with Different Solvents Extraction of Citric Acid and Maleic Acid from Their Aqueous Solutions Using a Phosphorus-Bonded Extractant, Tri-n-octylphosphineoxide, and a Secondary Amine, Dioctylamine Liquid-Liquid Equilibrium Data and Correlation for the Quaternary Systems Water + Polyphenol (Hydroquinone, Catechol, and Resorcinol) + Methyl Isobutyl Ketone + Methylbenzene Liquid-Liquid Equilibria for the Ternary Systems Water + Cyclohexanol + Methyl Isobutyl Carbinol and Water + Cyclohexanol + Methyl Isobutyl Ketone at Different Temperatures Liquid-Liquid Equilibrium of Water + 2-Methoxyphenol + Methyl Isobutyl Ketone and Water + 1,2-Benzenediol + Methyl Isobutyl Ketone at 303.15 K and 328.15 K Solubility of 2,5-Furandicarboxylic Acid in Eight Pure Solvents and Two Binary Solvent Systems at 313.15-363.15 K Liquid-Liquid Equilibrium for the Ternary Systems (Methyl Isobutyl Ketone + Quinoline or Isoquinoline + Water) at 298.15, 318.15, and 338.15 K Liquid-Liquid Equilibrium for Ternary Systems, Water + 5-Hydroxymethylfurfural + (1-Butanol, Isobutanol, Methyl Isobutyl Ketone), at 313.15, 323.15, and 333.15 K Solubility Determination and Correlation of Glibenclamide in 11 Monosolvents and (Acetone + Acetonitrile) Binary Solvents from 283.15 K to 323.15 K Experimental Study on Reactive Extraction of Malonic Acid with Validation by Fourier Transform Infrared Spectroscopy Comparison of Extractability of Oxalic Acid from Dilute Aqueous Solutions Using Dioctylamine and Trioctylphosphine Oxide Effect of Diluents on Extraction Equilibrium of trans-Aconitic Acid Thermodynamic Studies of Solubility for Naphthalene in 12 Solvents from 279 to 330 K Parametric Analysis of Mandelic Acid Separation from Aqueous Solutions by Using Secondary Amine Mixture (Amberlite LA-2) in Various Diluents Experiments and COSMO-SAC Modeling of Methyl Isobutyl Ketone + Dimethylphenols + Water Mixtures Vapor-Liquid Equilibrium for Binary of 1-Butanol + N,N-Dimethylacetamide and Methyl Isobutyl Ketone + N,N-Dimethylacetamide at 101.3 kPa Air-Water Partitioning of C5 and C6 Alkanones: Measurement, Critical Compilation, Correlation, and Recommended Data Viscosities, Densities, and Speeds of Sound of Binary Mixtures of o-Xylene, m-Xylene, p-Xylene, and Isopropylbenzene with 4-Methylpentan-2-one at 298.15 K Measurements of Quaternary Liquid-Liquid Equilibrium for Water + Acetic Acid + Propionic Acid + Solvent (Butyronitrile, Benzyl Acetate, or Methyl Isobutyl Ketone) at 298.15 K Liquid-Liquid Equilibria for the Ternary System Methyl Isobutyl Ketone + Water + Hydroquinone Isobaric Vapor-Liquid Equilibria for Binary and Ternary Mixtures with Cyclohexane, Cyclohexene, and Methyl Isobutyl Ketone at 100 kPa Liquid-Liquid and Vapor-Liquid-Liquid Equilibrium of the 4-Methyl-2-pentanone + 2-Butanol + Water System Evaluation of the Performance of Four Solvents for the Liquid Liquid Extraction of Acrylic Acid from Water Biomass-Derived Platform Chemicals: Thermodynamic Studies on the Extraction of 5-Hydroxymethylfurfural from Ionic Liquids Liquid Liquid Equilibria for the System Water + 4-Methylpentan-2-one + Acetic Acid at Several Temperatures Vapor Liquid Equilibrium, Excess Molar Enthalpies, and Excess Molar Volumes of Binary Mixtures Containing Methyl Isobutyl Ketone (MIBK) and 2-Butanol, tert-Pentanol, or 2-Ethyl-1-hexanol Determination of Henry's Law Constants Using Internal Standards with Benchmark Values Liquid Liquid Equilibria for the Ternary System Methyl Isobutyl Ketone + m-Benzenediol + Water Liquid Liquid Equilibria for the Ternary System Methyl Isobutyl Ketone + 1,2-Benzenediol + Water Liquid Liquid Equilibria of the Ternary System Water + Acrylic Acid + 4-Methyl-2-pentanone at 298.15 K Liquid Liquid Equilibria for the Ternary System Water + Methyl Isobutyl Ketone + tert-Butyl Alcohol at Several Temperatures Viscosity of the Tributyl Phosphate + Methyl Isobutyl Ketone + Phosphoric Acid System Volumetric and Transport Properties of Binary Liquid Mixtures of Aliphatic Ketones with Phenylacetonitrile at T = 308.15 K Densities and Speeds of Sound for Binary Liquid Mixtures of Thiolane-l,l-dioxide with Butanone, Pentan-2-one, Pentan-3-one, and 4-Methyl-pentan-2-one at T = (303.15 or 308.15 or 313.15) K Excess Molar Enthalpies and Vapor-Liquid Equilibrium for N-Methyl-2-pyrrolidone with Ketones Solubility of Penicillin Sulfoxide in Different Solvents Comparison of the Efficiencies of Amine Extractants on Lactic Acid with Different Organic Solvents Conductivity and Dissociation Constants of Quaternary Ammonium Perchlorates and Picrates in 4-Methyl-pentan-2-one Joback Method KDB Aqueous Solubility Prediction Method Estimated Solubility Method McGowan Method NIST Webbook The Yaws Handbook of Vapor Pressure
Note: Cheméo is only indexing the data, follow the source links to retrieve the latest data. The source is also providing more information like the publication year, authors and more.
Take the time to validate and double check the source of the data.
Outlier