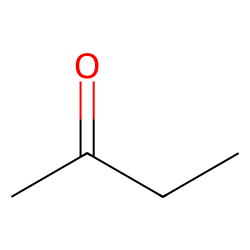
Physical Properties
Temperature Dependent Properties
Property
Value
Unit
Temperature (K)
Source
Cp,gas [113.43; 138.62]
J/mol×K
[347.15; 467.15]
Cp,gas 113.43 ± 0.23
J/mol×K
347.15
NIST
Cp,gas 115.65 ± 0.17
J/mol×K
358.79
NIST
Cp,gas 118.70 ± 0.18
J/mol×K
371.90
NIST
Cp,gas 119.03 ± 0.24
J/mol×K
372.15
NIST
Cp,gas 121.75 ± 0.18
J/mol×K
385.60
NIST
Cp,gas 124.39 ± 0.25
J/mol×K
397.15
NIST
Cp,gas 124.60 ± 0.19
J/mol×K
399.55
NIST
Cp,gas 126.98 ± 0.19
J/mol×K
410.70
NIST
Cp,gas 131.71 ± 0.26
J/mol×K
432.15
NIST
Cp,gas 138.62 ± 0.28
J/mol×K
467.15
NIST
Cp,liquid [157.91; 162.20]
J/mol×K
[293.00; 303.15]
Cp,liquid 158.00
J/mol×K
293.00
NIST
Cp,liquid 160.70
J/mol×K
297.00
NIST
Cp,liquid 160.70
J/mol×K
297.00
NIST
Cp,liquid 158.40
J/mol×K
298.10
NIST
Cp,liquid 159.20
J/mol×K
298.15
NIST
Cp,liquid 157.91
J/mol×K
298.15
NIST
Cp,liquid 158.40
J/mol×K
298.15
NIST
Cp,liquid 158.70
J/mol×K
298.15
NIST
Cp,liquid 158.40
J/mol×K
298.15
NIST
Cp,liquid 158.91
J/mol×K
298.15
NIST
Cp,liquid 158.41
J/mol×K
298.15
NIST
Cp,liquid 159.00
J/mol×K
298.15
NIST
Cp,liquid 162.20
J/mol×K
303.15
NIST
η [0.0002545; 0.0004306]
Pa×s
[288.15; 323.15]
η 0.0004306
Pa×s
288.15
Densiti...
η 0.0004306
Pa×s
288.15
Densiti...
η 0.0004136
Pa×s
293.15
Densiti...
η 0.0004136
Pa×s
293.15
Densiti...
η 0.0003980
Pa×s
293.15
Dynamic...
η 0.0003980
Pa×s
293.15
Physica...
η 0.0003956
Pa×s
293.15
Densiti...
η 0.0003810
Pa×s
298.15
Densiti...
η 0.0003962
Pa×s
298.15
Densiti...
η 0.0003856
Pa×s
298.15
Density...
η 0.0003856
Pa×s
298.15
Excess ...
η 0.0003780
Pa×s
298.15
Physica...
η 0.0002828
Pa×s
298.15
Densiti...
η 0.0003780
Pa×s
298.15
Dynamic...
η 0.0003962
Pa×s
298.15
Densiti...
η 0.0003620
Pa×s
303.15
Densiti...
η 0.0003600
Pa×s
303.15
Dynamic...
η 0.0003793
Pa×s
303.15
Densiti...
η 0.0003653
Pa×s
303.15
Density...
η 0.0003680
Pa×s
303.15
Densiti...
η 0.0003653
Pa×s
303.15
Excess ...
η 0.0003600
Pa×s
303.15
Physica...
η 0.0002666
Pa×s
303.15
Densiti...
η 0.0003793
Pa×s
303.15
Densiti...
η 0.0003440
Pa×s
308.15
Excess ...
η 0.0003520
Pa×s
308.15
Densiti...
η 0.0002601
Pa×s
308.15
Densiti...
η 0.0003440
Pa×s
308.15
Density...
η 0.0003626
Pa×s
308.15
Densiti...
η 0.0003450
Pa×s
308.15
Densiti...
η 0.0003626
Pa×s
308.15
Densiti...
η 0.0003461
Pa×s
313.15
Densiti...
η 0.0002545
Pa×s
313.15
Densiti...
η 0.0003461
Pa×s
313.15
Densiti...
η 0.0003300
Pa×s
313.15
Densiti...
η 0.0003390
Pa×s
313.15
Densiti...
η 0.0003303
Pa×s
318.15
Densiti...
η 0.0003303
Pa×s
318.15
Densiti...
η 0.0003160
Pa×s
318.15
Densiti...
η 0.0003030
Pa×s
323.15
Densiti...
η 0.0003167
Pa×s
323.15
Densiti...
η 0.0003168
Pa×s
323.15
Densiti...
Δfus H [8.38; 8.48]
kJ/mol
[186.10; 186.50]
Δfus H 8.48
kJ/mol
186.10
NIST
Δfus H 8.38
kJ/mol
186.47
NIST
Δfus H 8.44
kJ/mol
186.48
NIST
Δfus H 8.44
kJ/mol
186.50
NIST
Δfus H 8.44
kJ/mol
186.50
NIST
Δvap H [30.00; 35.60]
kJ/mol
[310.00; 505.00]
Δvap H 35.60
kJ/mol
310.00
NIST
Δvap H 33.80 ± 0.10
kJ/mol
314.00
NIST
Δvap H 33.80
kJ/mol
315.00
NIST
Δvap H 34.60
kJ/mol
318.00
NIST
Δvap H 32.30 ± 0.10
kJ/mol
338.00
NIST
Δvap H 33.90
kJ/mol
339.00
NIST
Δvap H 33.90
kJ/mol
342.00
NIST
Δvap H 31.30 ± 0.10
kJ/mol
352.00
NIST
Δvap H 31.21
kJ/mol
352.60
KDB
Δvap H 31.30
kJ/mol
352.80
NIST
Δvap H 30.50 ± 0.10
kJ/mol
363.00
NIST
Δvap H 30.00 ± 0.10
kJ/mol
370.00
NIST
Δvap H 32.50
kJ/mol
378.00
NIST
Δvap H 31.60
kJ/mol
438.00
NIST
Δvap H 31.10
kJ/mol
505.00
NIST
Pvap [15.74; 101.30]
kPa
[303.15; 352.72]
Pvap 15.74
kPa
303.15
Density...
Pvap 20.00
kPa
309.65
Measure...
Pvap 30.00
kPa
319.95
Measure...
Pvap 94.00
kPa
350.25
Vapor L...
Pvap 101.30
kPa
352.68
Measure...
Pvap 101.30
kPa
352.72
Isobari...
Pvap 101.30
kPa
352.72
Isobari...
n 0 [1.36294; 1.38153]
[288.15; 323.15]
n 0 1.38153
288.15
Experim...
n 0 1.37870
293.15
Solubil...
n 0 1.37900
293.15
Solid-L...
n 0 1.37910
293.15
Isother...
n 0 1.37879
293.15
Mixing ...
n 0 1.37880
293.15
Solubil...
n 0 1.37880
293.15
Isother...
n 0 1.37879
293.15
Thermod...
n 0 1.37892
293.15
Experim...
n 0 1.37631
298.15
Experim...
n 0 1.37700
298.15
Excess ...
n 0 1.37618
298.15
Ternary...
n 0 1.37930
298.15
Isobari...
n 0 1.37618
298.15
Mixing ...
n 0 1.37618
298.15
Thermod...
n 0 1.37500
298.15
Densiti...
n 0 1.37690
298.15
(Vapor ...
n 0 1.37604
298.15
Propert...
n 0 1.37614
298.15
Isobari...
n 0 1.37623
298.15
Vapor l...
n 0 1.37690
298.15
Bubble-...
n 0 1.37623
298.15
Densiti...
n 0 1.37610
298.15
Isobari...
n 0 1.37355
303.15
Thermod...
n 0 1.37366
303.15
Experim...
n 0 1.37383
303.15
Density...
n 0 1.37355
303.15
Mixing ...
n 0 1.37000
308.15
Densiti...
n 0 1.37102
308.15
Experim...
n 0 1.36834
313.15
Experim...
n 0 1.36570
318.15
Densiti...
n 0 1.36565
318.15
Experim...
n 0 1.36294
323.15
Experim...
ρl [772.97; 810.33]
kg/m3
[288.15; 323.15]
ρl 810.33
kg/m3
288.15
Excess ...
ρl 810.26
kg/m3
288.15
Volumet...
ρl 805.00
kg/m3
293.00
KDB
ρl 799.80
kg/m3
293.15
(Vapour...
ρl 805.06
kg/m3
293.15
Thermod...
ρl 805.06
kg/m3
293.15
Volumet...
ρl 808.64
kg/m3
293.15
Volumet...
ρl 804.90
kg/m3
293.15
Speed o...
ρl 804.97
kg/m3
293.15
Speed o...
ρl 805.17
kg/m3
293.15
Speed o...
ρl 804.83
kg/m3
293.15
Speed o...
ρl 799.94
kg/m3
298.15
Speed o...
ρl 799.68
kg/m3
298.15
Speed o...
ρl 799.74
kg/m3
298.15
Speed o...
ρl 799.75
kg/m3
298.15
Excess ...
ρl 802.03
kg/m3
298.15
Quatern...
ρl 799.97
kg/m3
298.15
Thermod...
ρl 799.97
kg/m3
298.15
Thermod...
ρl 799.60
kg/m3
298.15
Volumet...
ρl 800.30
kg/m3
298.15
Liquid-...
ρl 799.78
kg/m3
298.15
Thermod...
ρl 799.70
kg/m3
298.15
Isobari...
ρl 799.97
kg/m3
298.15
Thermod...
ρl 800.87
kg/m3
298.15
Experim...
ρl 799.83
kg/m3
298.15
Volumet...
ρl 799.62
kg/m3
298.15
Excess ...
ρl 803.24
kg/m3
298.15
Volumet...
ρl 799.97
kg/m3
298.15
Thermod...
ρl 799.60
kg/m3
298.15
Speed o...
ρl 794.67
kg/m3
303.15
Speed o...
ρl 794.47
kg/m3
303.15
Speed o...
ρl 794.41
kg/m3
303.15
Speed o...
ρl 794.55
kg/m3
303.15
Thermod...
ρl 794.63
kg/m3
303.15
Excess ...
ρl 794.60
kg/m3
303.15
Viscome...
ρl 802.50
kg/m3
303.15
Effect ...
ρl 799.71
kg/m3
303.15
Excess ...
ρl 797.88
kg/m3
303.15
Volumet...
ρl 794.50
kg/m3
303.15
Excess ...
ρl 794.60
kg/m3
303.15
Volumet...
ρl 799.69
kg/m3
303.15
Studies...
ρl 794.57
kg/m3
303.15
Volumet...
ρl 794.33
kg/m3
303.15
Speed o...
ρl 789.38
kg/m3
308.15
Speed o...
ρl 789.12
kg/m3
308.15
Speed o...
ρl 789.18
kg/m3
308.15
Speed o...
ρl 789.27
kg/m3
308.15
Volumet...
ρl 792.45
kg/m3
308.15
Volumet...
ρl 800.10
kg/m3
308.15
Effect ...
ρl 789.04
kg/m3
308.15
Speed o...
ρl 783.85
kg/m3
313.15
Speed o...
ρl 783.80
kg/m3
313.15
Speed o...
ρl 784.01
kg/m3
313.15
Excess ...
ρl 784.00
kg/m3
313.15
Volumet...
ρl 800.30
kg/m3
313.15
Effect ...
ρl 784.00
kg/m3
313.15
Viscome...
ρl 783.95
kg/m3
313.15
Volumet...
ρl 786.94
kg/m3
313.15
Volumet...
ρl 783.72
kg/m3
313.15
Speed o...
ρl 778.44
kg/m3
318.15
Speed o...
ρl 778.48
kg/m3
318.15
Speed o...
ρl 778.36
kg/m3
318.15
Speed o...
ρl 773.04
kg/m3
323.15
Speed o...
ρl 773.09
kg/m3
323.15
Speed o...
ρl 773.30
kg/m3
323.15
Viscome...
ρl 773.30
kg/m3
323.15
Volumet...
ρl 772.97
kg/m3
323.15
Speed o...
Δfus S [44.98; 45.59]
J/mol×K
[186.10; 186.48]
Δfus S 45.59
J/mol×K
186.10
NIST
Δfus S 44.98
J/mol×K
186.47
NIST
Δfus S 45.25
J/mol×K
186.48
NIST
csound,fluid [1136.00; 1212.30]
m/s
[293.15; 313.15]
csound,fluid 1212.30
m/s
293.15
Thermod...
csound,fluid 1212.00
m/s
293.15
Thermod...
csound,fluid 1192.00
m/s
298.15
Vapor l...
csound,fluid 1191.00
m/s
298.15
Thermod...
csound,fluid 1190.80
m/s
298.15
Thermod...
csound,fluid 1170.80
m/s
303.15
Thermod...
csound,fluid 1170.60
m/s
303.15
Thermod...
csound,fluid 1178.00
m/s
303.15
Densiti...
csound,fluid 1158.00
m/s
308.15
Densiti...
csound,fluid 1136.00
m/s
313.15
Densiti...
γ [0.02; 0.02]
N/m
[298.15; 298.20]
γ 0.02
N/m
298.15
Concent...
γ 0.02
N/m
298.20
KDB
λ [0.13; 0.15]
W/m×K
[284.48; 374.56]
λ 0.15
W/m×K
284.48
Thermal...
λ 0.15
W/m×K
294.42
Thermal...
λ 0.15
W/m×K
305.02
Thermal...
λ 0.14
W/m×K
314.26
Thermal...
λ 0.14
W/m×K
323.96
Thermal...
λ 0.14
W/m×K
334.71
Thermal...
λ 0.14
W/m×K
344.54
Thermal...
λ 0.13
W/m×K
354.33
Thermal...
λ 0.13
W/m×K
364.46
Thermal...
λ 0.13
W/m×K
374.56
Thermal...
Datasets
Viscosity, Pa*s
Fixed
Measured
Temperature, K - Liquid
Pressure, kPa - Liquid
Viscosity, Pa*s - Liquid
308.15
101.30
0.0003
Reference
Correlations
Similar Compounds
Find more compounds similar to 2-Butanone .
Mixtures
Diethyl carbonate + 2-Butanone
2-Butanone + Benzene, 1,3-dichloro-
1-Propanol + 2-Butanone + Benzene, 1,3-dichloro-
2-Butanone + 1-Butanol + Benzene, 1,3-dichloro-
2-Butanone + Benzene, 1,3-dichloro- + 1-Pentanol
2-Butanone + Benzene, 1,3-dichloro- + 1-Hexanol
2-Butanone + Formamide, N,N-dimethyl-
2-Butanone + Ethanol
2-Butanone + Pentane, 2,2,4-trimethyl-
2-Butanone + Ethanol + Pentane, 2,2,4-trimethyl-
1-Propanol + 2-Butanone
2-Butanone + Isopropyl Alcohol
n-Hexane + 2-Butanone
2-Butanone + Heptane
2-Butanone + 1-Propanamine, N-propyl-
2-Butanone + 1-Butanamine, N-butyl-
2-Butanone + Triethylamine
2-Butanone + Methylphosphonic acid
2-Butanone + Propanoic acid, 2-hydroxy-, ethyl ester
2-Butanone + Tetrahydrofuran
Find more mixtures with 2-Butanone .
Sources
KDB Pure (Korean Thermophysical Properties Databank) KDB Vapor Pressure Data Crippen Method A relative headspace method for Henry s constants of volatile organic compounds Vapor liquid equilibria for systems of diethyl carbonate and ketones and determination of group interaction parameters for the UNIFAC and ASOG methods Excess molar volumes of ternary mixtures of 1,3-dichlorobenzene and methyl ethyl ketone with 1-alkanols at 303.15K Experimental isobaric vapour liquid liquid equilibrium data for the quaternary systems water (1) ethanol (2) acetone (3) n-butyl acetate (4) and water (1) ethanol (2) acetone (3) methyl ethyl ketone (4) and their partially miscible-constituent ternaries (Vapor + liquid) equilibrium of binary mixtures formed by N,N-dimethyl formamide with some compounds at 95.1 kPa Liquid liquid equilibria of 1,3-dimethylimidazolium methyl sulfate with ketones, dialkyl carbonates and acetates Measurement of vapor.liquid equilibria (VLE) and excess enthalpies (HE) of binary systems with 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide and prediction of these properties and A using modified UNIFAC (Dortmund) Vapor liquid equilibria for binary and ternary mixtures of ethanol, 2-butanone, and 2,2,4-trimethylpentane at 101.3 kPa Effect of temperature on ultrasonic velocity and thermodynamic parameters of bisphenol-C-formaldehyde-acrylate resin solutions Isobaric vapour liquid equilibria for binary systems of 2-butanone with ethanol, 1-propanol, and 2-propanol at 20 and 101.3 kPa Investigation on isobaric vapor liquid equilibrium for acetic acid +water +methyl ethyl ketone + isopropyl acetate Compact apparatus for rapid measurement of high-pressure phase equilibria of carbon dioxide expanded liquids Activity coefficients at infinite dilution of organic solutes in the ionic liquid 1-ethyl-3-methylimidazolium methanesulfonate Measurements and correlation of vapour liquid equilibria of 2-butanone and hydrocarbons binary systems at two different pressures Solubility of lansoprazole in different solvents Thermodynamics of ketone + amine mixtures. Part IX. Excess molar enthalpies at 298.15K for dipropylamine, or dibutylamine + 2-alkanone systems and modeling of linear or aromatic amine + 2-alkanone mixtures in terms of DISQUAC and ERAS Thermodynamics of ketone + amine mixtures. Part X. Excess molarenthalpies at 298.15 K for N,N,N-triethylamine + 2-alkanone systems.Characterization of tertiary amine + 2-alkanone, and ofamino-ketone + n-alkane mixtures in terms of DISQUAC Liquid-liquid equilibria for the pseudo-ternary system {aqueous sulfuric acid solution + methyl ethyl ketone or methyl isopropyl ketone + phosphonium-based ionic liquids} at 298.15 K and atmospheric pressure Determination and correlation of solubilities of 1,2-bis(2-oxo-5,5-dimethyl-1,3,2-dioxyphosphacyclohexyl-2-imino) ethane in selected solvents Measurement and correlation of the solubilities of tetra(5,5-dimethyl-1,3-dioxaphosphorinanyl-2-oxy) neopentane in different pure solvents Experimental and theoretical study of interaction between organic compounds and 1- (4-sulfobutyl)-3-methylimidazolium based ionic liquids Study of interaction between organic compounds and mono or dicationic oxygenated ionic liquids using gas chromatography Solubility of methylphosphonic acid in selected organic solvents Experimental measurements and modelling of volumetric properties, refractive index and viscosity of binary systems of ethyl lactate with methyl ethyl ketone, toluene and n-methyl-2-pirrolidone at 288.15 323.15 K and atmospheric pressure. New UNIFAC VISCO and ASOG VISCO interaction parameters Activity coefficients at infinite dilution for different alcohols and ketones in [EMpy][ESO4]: Experimental data and modeling with PC-SAFT Densities, viscosities and refractive indices of binary mixtures containing methyl ethyl ketone. Friction theory. New UNIFAC-VISCO and ASOG-VISCO parameter determination Isobaric vapor-liquid equilibrium for methanol + methyl ethyl ketone + bis(trifluoromethylsulfonyl)imide-based ionic liquids at 101.3 kPa The solubility of maleic acid in various inorganic salts and organic solvents: Thermodynamics model and parameters Isobaric vapor-liquid equilibrium for 2-butanone + ethanol + phosphate-based ionic liquids at 101.3 kPa Measurements of activity coefficient at infinite dilution for organic solutes in tetramethylammonium chloride + ethylene glycol deep eutectic solvent using gas-liquid chromatography Quaternary isothermal vapor-liquid equilibrium of the model biofuel 2-butanone + n-heptane + tetrahydrofuran + cyclohexane using Raman spectroscopic characterization Solubility of 1-methyl-4-nitropyrazole in seventeen pure solvents at temperatures from 283.15 K to 323.15 K Tetramethylammonium chloride + glycerol deep eutectic solvent as separation agent for organic liquid mixtures: Assessment from experimental limiting activity coefficients Measurements of activity coefficients at infinite dilution for organic solutes in two quaternary ammonium-based ionic liquids [DDA][ClO4] and [DDA][BF4] Application of trihexyltetradecylphosphonium dicyanamide ionic liquid for various types of separations problems: Activity coefficients at infinite dilution measurements utilizing GLC method A thermodynamic study of the ketoreductase-catalyzed reduction of 2-alkanones in non-aqueous solvents Thermodynamics of binary mixtures of N-methyl-2-pyrrolidinone and ketone. Experimental results and modelling of the solid-liquid equilibrium and vapou-liquid equilibrium. The Modified UNIFAC (Do) model characterization Systems with ionic liquids: Measurement of VLE and c1 data and prediction of their thermodynamic behavior using original UNIFAC, mod. UNIFAC(Do) and COSMO-RS(Ol) Properties of ionic liquid HMIMPF6 with carbonates, ketones and alkyl acetates Physical properties of the binary systems methylcyclopentane with ketones (acetone, butanone and 2-pentanone) at T = (293.15, 298.15, and 303.15) K. New UNIFAC-VISCO interaction parameters (Vapour + liquid) equilibria for (2-ethoxypropene + acetone) and (2-ethoxypropene + butanone) Excess molar enthalpies and volumes of binary mixtures of nonafluorobutylmethylether with ketones at T = 298.15 K Measurement of activity coefficients at infinite dilution in 1-hexadecyl-3-methylimidazolium tetrafluoroborate ionic liquid Mixing properties of binary mixtures presenting azeotropes at several temperatures Excess enthalpies and (vapour + liquid) equilibrium data for the binary mixtures of dimethylsulphoxide with ketones Density, viscosity, refractive index, excess molar enthalpy, viscosity, and refractive index deviations for the (1-butanol + 2-butanone) binary system at T = 303 K. A new adiabatic calorimeter for heat of mixing Excess molar volumes and ultrasonic studies of dimethylsulphoxide with ketones at T = 303.15 K Measurement and correlation of (liquid + liquid) equilibrium of the azeotrope (cyclohexane + 2-butanone) with different ionic liquids at T = 298.15 K Excess molar volumes and ultrasonic studies of N-methyl-2-pyrrolidone with ketones at T = 303.15 K Activity coefficients at infinite dilution for solutes in the trioctylmethylammonium bis(trifluoromethylsulfonyl)imide ionic liquid using gas liquid chromatography Excess parameter studies on the binary mixtures of toluene with ketones at different temperatures Activity coefficients at infinite dilution of organic solutes in the ionic liquid trihexyl(tetradecyl)phosphonium tetrafluoroborate using gas liquid chromatography at T = (313.15, 333.15, 353.15, and 373.15) K Activity coefficients at infinite dilution of organic solutes in the ionic liquid 1-butyl-3-methylimidazolium hexafluoroantimonate using gas liquid chromatography at T = (313.15, 323.15, and 333.15) K Activity coefficients at infinite dilution of organic solutes in the ionic liquid, methyl(trioctyl)ammonium thiosalicylate, [N1888][TS] by gas liquid chromatography at T = (303.15, 313.15, and 323.15) K A volumetric and viscosity study for the binary mixtures of 1-hexyl-3-methylimidazolium tetrafluoroborate with some molecular solvents Interactions of volatile organic compounds with the ionic liquid 1-ethyl-3-methylimidazolium tetracyanoborate Combined effects of salinity and temperature on the solubility of organic compounds Activity coefficients at infinite dilution of organic solutes in the ionic liquid trihexyltetradecylphosphonium hexafluorophosphate using gas liquid chromatography at T = (313.15, 333.15, 353.15, and 363.15) K Volumetric properties of binary mixtures of N-ethylformamide with tetrahydrofuran, 2-butanone and ethylacetate from (293.15 to 313.15) K Interactions of volatile organic compounds with the ionic liquids 1-butyl-1-methylpyrrolidinium tetracyanoborate and 1-butyl-1-methylpyrrolidinium bis(oxalato)borate Activity coefficients at infinite dilution of organic solutes in the ionic liquid trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl)imide using gas-liquid chromatography at T = (313.15, 333.15, 353.15 and 373.15) K Infinite dilution activity coefficients of volatile organic compounds in two ionic liquids composed of the tris(pentafluoroethyl) trifluorophosphate ([FAP]) anion and a functionalized cation Measurement and correlation of solubility of dodecanedioic acid in different pure solvents from T = (288.15 to 323.15) K Thermodynamics of (ketone + amine) mixtures. Part XI. Excess molar enthalpies at T = 298.15 K for the (1-propanol + N,N,N-triethylamine + 2-butanone) system Measurement of activity coefficients at infinite dilution of organic solutes in the ionic liquid 1-ethyl-3-methylimidazolium 2-(2-methoxyethoxy) ethylsulfate at T = (308.15, 313.15, 323.15 and 333.15) K using gas + liquid chromatography Volumetric and optical properties for some (2-butanone + chloroalkane) binary mixtures at T = 298.15 K Activity coefficients at infinite dilution of organic solutes in methylphosphonate based ionic liquids using gas-liquid chromatography Isobaric (vapor + liquid) equilibria for the binary systems (1-methoxy-2- propanol + 2-methoxyethanol), (2-butanone + 2-methoxyethanol) and (water + 2-methoxyethanol) at pressures of (74.5, 101.3, and 134.0) kPa Screening of environmental friendly ionic liquid as a solvent for the different types of separations problem: Insight from activity coefficients at infinite dilution measurement using (gas + liquid) chromatography technique Thermodynamics of amide + ketone mixtures. 1. Volumetric, speed of sound and refractive index data for N,N-dimethylformamide + 2-alkanone systems at several temperatures Phase equilibria of the ternary systems of (water + diethyl carbonate) with acetone, or 2-butanone at four temperatures Thermodynamic study of the solubility of 2,4'-dihydroxydiphenyl sulfone in nine organic solvents from T = (278.15 to 313.15) K and thermodynamic properties of dissolution Solubility determination and thermodynamic modelling of terephthaldialdehyde in ten organic solvents from T = (273.15 to 318.15) K and mixing properties of solutions Measurement and modelling of econazole nitrate in twelve pure organic solvents at temperatures from 278.15 K to 318.15 K Solubility measurement and correlation of the form A of ibrutinib in organic solvents from 278.15 to 323.15 K Thermodynamic modelling for solubility of 4-nitrobenzaldehyde in different solvents at temperature range from (273.15 to 313.15) K and mixing properties of solutions Solubility determination and thermodynamic modelling of 3-amino-1,2,4-triazole in ten organic solvents from T = 283.15 K to T = 318.15 K and mixing properties of solutions Solubility measurement, model evaluation and thermodynamic analysis of rivaroxaban polymorphs in organic solvents Thermodynamic modelling for solubility of 5-chloro-1-methyl-4-nitroimidazole in eleven organic solvents from T = (283.15 to 318.15) K Solubility and solution thermodynamics of 2-methyl-4-nitroaniline in eleven organic solvents at elevated temperatures Solubility determination and modelling of 4-nitro-1,2-phenylenediamine in eleven organic solvents from T = (283.15 to 318.15) K and thermodynamic properties of solutions Solubility determination and thermodynamic functions of 2-chlorophenothiazine in nine organic solvents from T = 283.15 K to T = 318.15 K and mixing properties of solutions Solubility and thermodynamic functions of tebuconazole in nine organic solvents from T = (278.15 to 313.15) K and mixing properties of solutions Solubility of 2-isopropylimidazole in nine pure organic solvents and liquid mixture of (methanol + ethyl acetate) from T = (278.15 to 313.15) K: Experimental measurement and thermodynamic modelling Solubility of 4-methyl-2-nitroaniline in fourteen organic solvents from T = (278.15 to 313.15) K and mixing properties of solutions Thermodynamic functions for solubility of 3-nitro-o-toluic acid in nine organic solvents from T = (283.15 to 318.15) K and apparent thermodynamic properties of solutions Solubility modelling and dissolution properties of 5-phenyltetrazole in thirteen mono-solvents and liquid mixtures of (methanol + ethyl acetate) at elevated temperatures Thermodynamic functions for solubility of 2-mercaptobenzothiazole in eleven pure organic solvents at temperatures from 273.15 K to 318.15 K and mixing properties of solutions Separation of (water/butan-1-ol) binary systems based on activity coefficients at infinite dilution with phosphonium ionic liquid Studies of viscosities of dilute solutions of alkylamine in non-electrolyte solvents. II. Haloalkanes and other polar solvents Molar excess enthalpies of ethyl acetate + alkanols at T=298.15K, p=10MPa Thermodynamics of ketone + amine mixtures Part IV. Volumetric and speed of sound data at (293.15; 298.15 and 303.15 K) for 2-butanone +dipropylamine, +dibutylamine or +triethylamine systems Experimental Determination and Modeling of Densities, Refractive Indices of the Binary Mixtures of Dimethylphthalate (or Dimethyladipate) + 1-Butanol, or + 2-Butanol, or + 2-Butanone at T = (288.15 to 323.15) K Excess molar volumes of (1-chlorobutane +heptane + 2-butanone or 2-pentanone) at 288.15 , 303.15 and 313.15 K . Measurements and correlations. Densities and viscosities of binary mixtures of ethylmethylketone and 2-alkanols; application of the ERAS model and cubic EOS Volumetric properties of binary liquid mixtures of ketones with chloroalkanes at different temperatures and atmospheric pressure Isobaric Vapor Liquid Equilibrium for the Binary Systems of sec-Butyl Acetate + Methyl Ethyl Ketone, 2-Methoxyethanol, or 1,2-Dimethoxyethane at 101.3 kPa Solubility of Benzoic Acid and Aspirin in Pure Solvents Using Focused Beam Reflective Measurement Vapor Liquid Equilibria Measurements for the Five Linear C6 Esters with n-Octane Solubility Measurement and Thermodynamic Modeling of 4-Nitrophthalimide in Twelve Pure Solvents at Elevated Temperatures Ranging from (273.15 to 323.15) K Isobaric Vapor Liquid Liquid Phase Equilibria Measurements of Three Ternary Water + 2-Butanone + Aliphatic Alcohol (Ethanol, 1-Propanol, 2-Propanol) Systems at 101.3 kPa Solubility Measurement and Correlation of Two Erlotinib Hydrochloride Polymorphs in Alcohols and Ketones from 273.15 to 323.15 K Experimental and Modeling Studies on the Solubility of 2-Chloro-N-(4-methylphenyl)propanamide (S1) in Binary Ethyl Acetate + Hexane, Toluene + Hexane, Acetone + Hexane, and Butanone + Hexane Solvent Mixtures Using Polythermal Method Thermodynamic Functions for Solubility of 1-Hydroxybenzotriazole in Sixteen Solvents at Temperatures from (278.15 to 313.15) K and Mixing Property of Mixtures Solubility Modeling and Mixing Thermodynamics of Thiamphenicol in Water and Twelve Neat Organic Solvents from T = (278.15 to 318.15) K Extraction of Citric Acid and Maleic Acid from Their Aqueous Solutions Using a Phosphorus-Bonded Extractant, Tri-n-octylphosphineoxide, and a Secondary Amine, Dioctylamine Isobaric Vapor-Liquid Equilibrium for 2-Butanone + Ethanol System Containing Different Ionic Liquids at 101.3 kPa Solubility Measurement and Thermodynamic Modeling of N-(4-Methylphenyl-Z-3-chloro-2-(phenylthio)propenamide in 12 Pure Solvents at Temperatures Ranging from 278.15 to 318.15 K Solubility of 2-Chlorophenylacetic Acid in 12 Pure Solvents from T = (273.15/283.15 to 318.15) K: Determination and Modeling Solubility and Molecular Interactions of Trimetazidine Hydrochloride in 12 Monosolvents and Solvent Mixtures of Methanol + (Ethanol, N,N-Dimethylformamide or Ethyl Acetate) Solubility Modeling and Solvent Effects of Allopurinol in 15 Neat Solvents Isothermal Vapor-Liquid Equilibrium Measurements of Butan-2-one + 2-Methyl-propan-1-ol/Pentan-1-ol Activity Coefficients at Infinite Dilution of Various Solutes in Tetrapropylammonium Bromide + 1,6-Hexanediol Deep Eutectic Solvent Measurement and Correlation of Solubility and Thermodynamic Properties of Vinpocetine in Nine Pure Solvents and (Ethanol + Water) Binary Solvent Measurement and Correlation of Solubility of Gatifloxacin in 12 Pure Solvents from 273.15 K to 318.15 K Solubility Measurement and Thermodynamic Model Correlation and Evaluation of 2-Chloro-5-nitroaniline in 12 Pure Solvents Solubility Modeling and Solvent Effect of 2-Amino-6-chloropurine in Twelve Neat Solvents Solubility in Different Solvents, Correlation, and Solvent Effect in the Solvent Crystallization Process of Iohexol Solubility Modeling and Solvent Effect for Flubendazole in 12 Neat Solvents Solubility, Model Correlation, and Solvent Effect of 2-Amino-3-methylbenzoic Acid in 12 Pure Solvents Solid-Liquid Equilibrium Measurements for Posaconazole and Voriconazole in Several Solvents between T = 278.2 and 323.2 K Using Differential Thermal Analysis/Thermal Gravimetric Analysis Solubility Determination and Thermodynamic Modeling of 2-Mercaptobenzimidazole in 12 Solvents from T = 278.15 K to T = 318.15 K o-Nitrophenylacetonitrile Solubility in Several Pure Solvents: Measurement, Correlation, and Solvent Effect Analysis Assessment of Pyrrolidinium-Based Ionic Liquid for the Separation of Binary Mixtures Based on Activity Coefficients at Infinite Dilution Solubility Determination and Thermodynamic Mixing Properties of 5-Methyl-2-pyrazinecarboxylic Acid in Different Solvents Solubility and Thermodynamic Modeling of Dimethyl Terephthalate in Pure Solvents and the Evaluation of the Mixing Properties of the Solutions Solubility and Data Correlation of b-Arbutin in Different Monosolvents from 283.15 to 323.15 K Solubility Data for Roflumilast and Maraviroc in Various Solvents between T = (278.2-323.2) K Solid-Liquid Phase Equilibrium and Thermodynamic Analysis of N,N'-Diethylthiourea in Different Solvent Systems Measurement of Activity Coefficients of Solutes at Infinite Dilution in (Dimethyl Sulfoxide + Acetamide, or Formamide, or Urea) Using Gas Liquid Chromatography at the Temperature 298.15 K Bubble-Temperature Measurements on Some Binary Mixtures Formed by Tetrahydrofuran or Amyl Alcohol with Hydrocarbons, Chlorohydrocarbons, or Butanols at (94.6 or 95.8) kPa Measurement of Henry s Law Constants for Acetone, 2-Butanone, 2,3-Butanedione, and Isobutyraldehyde Using a Horizontal Flow Reactor Excess Molar Volumes and Surface Tensions of Xylene with Acetone or 2-Butanone at 298.15 K Densities and Viscosities of Binary Liquid Mixtures of Trichloroethylene and Tetrachloroethylene with Some Polar and Nonpolar Solvents Determination of Henry s Law Constants and Activity Coefficients at Infinite Dilution of Flavor Compounds in Water at 298 K with a Gas-Chromatographic Method Densities and Viscosities for Binary and Ternary Mixtures of Ethanol, 2-Butanone, and 2,2,4-Trimethylpentane at T = (298.15, 308.15, and 318.15) K Measurement of Isobaric Vapor - Liquid Equilibria of Dimethyl Carbonate with Acetone, 2-Butanone and 2-Pentanone at 101.3 kPa and Density and Speed of Sound at 298.15 K Viscosities, Densities, and Speeds of Sound of Binary Mixtures of o-Xylene, m-Xylene, p-Xylene, and Isopropylbenzene with 2-Butanone at 298.15 K Thermal Conductivity of Some Oxygenated Fuels and Additives in the Saturated Liquid Phase Measurement of Activity Coefficients at Infinite Dilution of Benzene, Toluene, Ethanol, Esters, Ketones, and Ethers at Various Temperatures in Water Using the Dilutor Technique Solubilities of Proxetine Hydrochloride Hemihydrate between 286 K and 363 K Concentration Dependence of Surface Tension for Very Dilute Aqueous Solutions of Organic Non-Electrolytes Dynamic Viscosities of the Binary Systems Cyclohexane and Cyclopentane with Acetone, Butanone, or 2-Pentanone at Three Temperatures T ) (293.15, 298.15, and 303.15) K Excess Molar Volumes and Viscosity Deviations of Binary Liquid Mixtures of 1,3-Dioxolane and 1,4-Dioxane with Butyl Acetate, Butyric Acid, Butylamine, and 2-Butanone at 298.15 K Activity Coefficients at Infinite Dilution in 1-Alkyl-3-methylimidazolium Tetrafluoroborate Ionic Liquids Thermodynamic Properties of Mixtures Containing Ionic Liquids: Activity Coefficients at Infinite Dilution of Organic Compounds in 1-Propyl Boronic Acid-3-Alkylimidazolium Bromide and 1-Propenyl-3-alkylimidazolium Bromide Using Inverse Gas Chromatography Henry s Constants of 1-Alkanols and 2-Ketones in Salt Solutions Density, Viscosity, Vapor-Liquid Equilibrium, Excess Molar Volume, Viscosity Deviation, and Their Correlations for the Chloroform + 2-Butanone Binary System Thermodynamic Properties of Ionic Liquids in Organic Solvents from (293.15 to 303.15) K Measurement and Correlation of Griseofulvin Solubility in Different Solvents at Temperatures from (281.95 to 357.60) K Solubility of Artemisinin in Different Single and Binary Solvent Mixtures Between (284.15 and 323.15) K and NRTL Interaction Parameters Solubilities of Phosphorus-Containing Compounds in Selected Solvents Liquid-Liquid and Vapor-Liquid-Liquid Equilibrium of the 2-Butanone + 2-Butanol + Water System Thermodynamics of Ketone + Amine Mixtures. Part III. Volumetric and Speed of Sound Data at (293.15, 298.15, and 303.15) K for 2-Butanone + Aniline, + N-Methylaniline, or + Pyridine Systems Activity Coefficients at Infinite Dilution in Methylimidazolium Nitrate Ionic Liquids Solubilities of N-[(4-Bromo-3,5-difluorine)-phenyl]maleimide in Different Organic Solvents Solubility of Candesartan Cilexetil in Different Solvents at Various Temperatures Viscometric Behavior of Binary Mixtures of Butan-2-one with Benzene at T = (303.15, 313.15, and 323.15) K Solubility of CO2 in Alcohols, Glycols, Ethers, and Ketones at High Pressures from (288.15 to 318.15) K Densities and Viscosities of the Binary Mixtures of Phenylmethanol with 2-Butanone Activity Coefficients at Infinite Dilution of Organic Compounds in Four New Imidazolium-Based Ionic Liquids Thermodynamics of Ketone + Amine Mixtures. Part VIII. Molar Excess Enthalpies at 298.15 K for n-Alkanone + Aniline or + N-Methylaniline Systems Partition Coefficients of Organic Compounds in Four New Tetraalkylammonium Bis(trifluoromethylsulfonyl)imide Ionic Liquids Using Inverse Gas Chromatography Interactions of Volatile Organic Compounds with the Ionic Liquid 1-Butyl-1-methylpyrrolidinium Dicyanamide Evaluation of the Performance of Trigeminal Tricationic Ionic Liquids for Separation Problems Activity Coefficients at Infinite Dilution for Organic Compounds Dissolved in 1-Alkyl-1-methylpyrrolidinium Bis(trifluoromethylsulfonyl)imide Ionic Liquids Having Six-, Eight-, and Ten-Carbon Alkyl Chains Determination of Henry's Law Constants Using Internal Standards with Benchmark Values Effect of Ionic Liquids on the Isobaric Vapor-Liquid equilibrium behavior of methanol-methyl ethyl ketone (MEK) Solubility of Veratric Acid in Eight Monosolvents and Ethanol + 1-Butanol at Various Temperatures Isothermal Vapor Liquid Equilibrium Data for the Butan-2-one + Methanol or Ethanol Systems Using a Static-Analytic Microcell Activity Coefficients at Infinite Dilution for Organic Solutes Dissolved in Three 1-Alkyl-1-methylpyrrolidinium Bis(trifluoromethylsulfonyl)imide Ionic Liquids Bearing Short Linear Alkyl Side Chains of Three to Five Carbons Infinite Dilution Activity Coefficients of Solutes Dissolved in Two Trihexyl(tetradecyl)phosphonium Ionic Liquids Speed of sound, density and related thermodynamic excess properties of binary mixtures of butan-2-one with C1-C4 nalkanols and chloroform Densities, Viscosities and Refractive Indices of the Ternary Mixture Dimethyladipate + 2-Butanone + 1-Butanol at T = (288.15 to 323.15) K Ternary Liquid-Liquid Equilibria Ethanol + 2-Butanone + 1-Butyl-3-methylimidazolium Hexafluorophosphate, 2-Propanol + 2-Butanone + 1-Butyl-3-methylimidazolium Hexafluorophosphate, and 2-Butanone + 2-Propanol + 1,3-Dimethylimidazolium Methyl Sulfate at 298.15 K Solubility of Imidacloprid in Different Solvents Isobaric Vapor Liquid Equilibria for Binary Systems of Acetone + Isopropenyl Acetate, 2-Butanone + Isopropenyl Acetate, and Isopropenyl Acetate + Acetylacetone at 101.3 kPa Solubility of Lovastatin in Ethyl Acetate, Propyl Acetate, Isopropyl Acetate, Butyl Acetate, sec-Butyl Acetate, Isobutyl Acetate, tert-Butyl Acetate, and 2-Butanone, between (285 and 313) K Measurement of Infinite Dilution Activity Coefficients of Alcohols, Ketones, and Aromatic Hydrocarbons in 4-Methyl-N-butylpyridinium Tetrafluoroborate and 1-Butyl-3-methylimidazolium Hexafluorophosphate by Gas-Liquid Chromatography Volumetric and Transport Properties of Binary Liquid Mixtures of Aliphatic Ketones with Phenylacetonitrile at T = 308.15 K Densities and Viscosities of Binary Liquid Mixtures of 2-Butanone with Branched Alcohols at (293.15 to 313.15) K Activity Coefficients at Infinite Dilution of Organic Compounds in 1-Butyl-3-methylimidazolium Tetrafluoroborate Using Inverse Gas Chromatography Activity Coefficients at Infinite Dilution of Organic Compounds in Trihexyl(tetradecyl)phosphonium Bis(trifluoromethylsulfonyl)imide Using Inverse Gas Chromatography Volumetric Behavior of the Binary Mixtures of Methyl Ethyl Ketone with n-Hexane, Cyclohexane, and Benzene at T ) (303.15, 313.15, and 323.15) K Densities and Speeds of Sound for Binary Liquid Mixtures of Thiolane-l,l-dioxide with Butanone, Pentan-2-one, Pentan-3-one, and 4-Methyl-pentan-2-one at T = (303.15 or 308.15 or 313.15) K Excess Molar Enthalpies and Vapor-Liquid Equilibrium for N-Methyl-2-pyrrolidone with Ketones Partition Coefficients of Organic Compounds in New Imidazolium and Tetralkylammonium Based Ionic Liquids Using Inverse Gas Chromatography Isobaric Vapor-Liquid Equilibria for Binary and Ternary Mixtures of Ethanol and 2-Propanol with 2-Butanone and Butyl Propionate at 101.3 kPa Density and Viscosity of Ketones with Toluene at Different Temperatures and at Atmospheric Pressure Excess Enthalpies of Dibromomethane with Acetone, 1,4-Dioxane, Pyridine, Diethyl Ether, Ethyl Methyl Ketone, and Tetrahydrofuran at 303.15 K Solubility of 1,1'-(Ethane-1,2-diyl)-bis(3-methylpyridinium) Dihexafluorophosphate in Different Solvents Solubilities of 1,1?-(Propane-1,3-diyl)-bis(3-methyl-1H-imidazolium-1-yl) Dihexafluorophosphate in Different Solvents Solubility of 1,1'-(Butane-1,4-diyl)-bis(3-methyl-1H-imidazolium-1-yl) Dihexafluorophosphate in Water, Acetophenone, Cyclohexanone, Acetylacetone, and 2-Butanone Study of Ether-, Alcohol-, or Cyano-Functionalized Ionic Liquids Using Inverse Gas Chromatography Densities, Viscosities, and Refractive Indices of Binary Mixtures of Methyl Ethyl Ketone + Pentanol Isomers at Different Temperatures Solubility of 1,1'-(Butane-1,4-diyl)-bis(3-methylpyridinium) Dihexafluorophosphate in Different Solvents from (288.15 to 333.15) K Joback Method KDB Aqueous Solubility Prediction Method Estimated Solubility Method McGowan Method NIST Webbook The Yaws Handbook of Vapor Pressure
Note: Cheméo is only indexing the data, follow the source links to retrieve the latest data. The source is also providing more information like the publication year, authors and more.
Take the time to validate and double check the source of the data.
Outlier