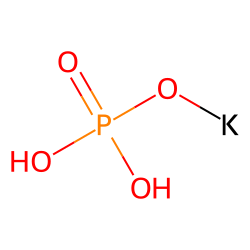
Similar Compounds
Find more compounds similar to Potassium phosphate, monobasic.
Mixtures
- Potassium phosphate, monobasic + Ammonium nitrate
- Xylose + Potassium phosphate, monobasic + Water
- D-Ribose + Potassium phosphate, monobasic + Water
- D-Fructose + Potassium phosphate, monobasic + Water
- Potassium phosphate, monobasic + Glucose + Water
- Potassium phosphate, monobasic + d-Mannose + Water
- Potassium phosphate, monobasic + Water + 2-Deoxy-D-glucose
- Sucrose + Potassium phosphate, monobasic + Water
- Potassium phosphate, monobasic + D-cellobiose + Water
- Potassium phosphate, monobasic + Maltose + Water
- Potassium phosphate, monobasic + Water
- Potassium phosphate, monobasic + Water + Potassium sulfate
- Potassium phosphate, monobasic + 1,2-Ethanediol + Water
- Potassium phosphate, monobasic + 1,2-Ethanediol
- potassium chloride + Potassium phosphate, monobasic + Water
- Potassium phosphate, monobasic + sodium chloride + Water
- Potassium phosphate, monobasic + Ethanol + Water
Sources
- Thermodynamic properties of binary aqueous solutions of orthophosphate salts, sodium, potassium and ammonium at T= 298.15 K
- Study of di-hydrogen (Na; K or NH4) orthophosphates in aqueous solutions at temperatures from 298.15 K to 353.15 K
- Solid-liquid phase equilibrium and phase diagram for the reciprocal quaternary system Na+, K+ // SO42,H2PO4- - H2O at 313.15 K
- Determination of related systems' solubility data (I) in the preparation process of K2SO4 or KH2PO4 by the solvent extraction method
- An investigation on the solid-liquid phase equilibrium of the quaternary system KH2PO4-H3PO4-CH2OHCH2OH-H2O
- Apparent molar volumes, apparent isentropic compressibilities, and viscosity B-coefficients of 1-ethyl-3-methylimidazolium bromide in aqueous di-potassium hydrogen phosphate and potassium di-hydrogen
- Evaluation of the impact of phosphate salts on the formation of ionic-liquid-based aqueous biphasic systems
- Comparative study of density, sound velocity and refractive index for (water + alkali metal) phosphates aqueous systems at T = (298.15, 303.15, and 308.15) K
- Thermodynamic modelling of high salinary phosphate solutions. I. Binary systems
- Thermodynamic modeling of high salinary phosphate solutions II. Ternary and higher systems
- pH effect on the enthalpy of dilution and volumetric properties of protocatechuic acid at T = 298.15 K
- Stable (solid + liquid) phase equilibrium for the ternary systems (K2SO4 + KH2PO4 + H2O), (K2SO4 + KCl + H2O) at T = 313.15 K
- Solubility and distribution of bicycle derivatives of 1,3-selenazine in pharmaceutically relevant media by saturation shake-flask method
- Probing the binding ability of vitamin B1 with bovine serum albumin: Calorimetric, light scattering, spectroscopic and volumetric studies
- Influence of selected potassium salts on thermal stability of ammonium nitrate
- Solid Liquid Phase Equilibrium for the Ternary System (Potassium Chloride + Potassium Dihydrogen Phosphate + Water) at (298.15 and 313.15) K
- Phase Equilibrium in the Aqueous Ternary System KH2PO4+ KCl +H2O at (288.15 and 303.15) K
- Solid Liquid Equilibria in the Quaternary System K+//H2PO42 , SO42 ,Cl H2O at 298.2 K and 323.2 K
- Viscosities of Some Saccharides in Aqueous Solutions of Phosphate-Based Inorganic Salts
- Phase Equilibrium for the Ternary Systems (KCl + K2SO4 + H2O) and (KH2PO4 + K2SO4 + H2O) at 288.15 K and Atmospheric Pressure
- Phase Equilibrium in the Aqueous Ternary System KH2PO4 NaH2PO4 H2O at 288.15 and 318.15 K
- Phase equilibrium in the System KH2PO4 + KCl + H3PO4 at 298.15 K and 308.15 K
- Solid-Liquid Phase Equilibria of the KH2PO4-KCl-H3PO4-H2O and KH2PO4-KCl-C2H5OH-H2O Systems at T = (298.15 and 313.15) K
- Osmotic and Activity Coefficient of 1-Ethyl-3-methylimidazolium Bromide in Aqueous Solutions of Potassium Dihydrogen Phosphate, Dipotassium Hydrogen Phosphate, and Tripotassium Phosphate at T = 298.15 K
- Ionic Liquid Based Aqueous Biphasic Systems with Controlled pH: The Ionic Liquid Cation Effect
- Solid Liquid Phase Equilibrium for the Ternary System K2SO4 + KH2PO4 + H2O at (298.15 and 333.15) K
- Solubility of KH2PO4 in KCl, H3PO4, and Their Mixture Solutions
- Phase Equilibrium for the Ternary System KH2PO4 + NaH2PO4 + H2O at 303.15 K
- NIST Webbook
Note: Cheméo is only indexing the data, follow the source links to retrieve the latest data. The source is also providing more information like the publication year, authors and more. Take the time to validate and double check the source of the data.