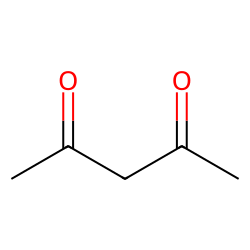
Physical Properties
Property
Value
Unit
Source
PAff 873.50
kJ/mol
NIST
BasG 836.80
kJ/mol
NIST
Δc H°liquid [-2687.00; -2581.90]
kJ/mol
Δc H°liquid -2685.40 ± 0.80
kJ/mol
NIST
Δc H°liquid -2667.00 ± 12.00
kJ/mol
NIST
Δc H°liquid -2687.00 ± 1.50
kJ/mol
NIST
Δc H°liquid -2581.90
kJ/mol
NIST
Δf G° -266.62
kJ/mol
Joback Calculated Property
Δf H°gas [-420.10; -376.10]
kJ/mol
Δf H°gas -384.40 ± 1.30
kJ/mol
NIST
Δf H°gas -420.10
kJ/mol
NIST
Δf H°gas -376.10 ± 2.00
kJ/mol
NIST
Δf H°liquid [-528.94; -414.10]
kJ/mol
Δf H°liquid -427.60 ± 1.10
kJ/mol
NIST
Δf H°liquid -447.30 ± 8.00
kJ/mol
NIST
Δf H°liquid -414.10 ± 2.00
kJ/mol
NIST
Δf H°liquid -528.94
kJ/mol
NIST
Δfus H° 11.90
kJ/mol
Joback Calculated Property
Δvap H° [41.78; 43.20]
kJ/mol
Δvap H° 41.78
kJ/mol
NIST
Δvap H° 43.20 ± 0.10
kJ/mol
NIST
Δvap H° 43.20 ± 1.00
kJ/mol
NIST
IE [8.82; 9.63]
eV
IE 8.85 ± 0.02
eV
NIST
IE 8.85 ± 0.05
eV
NIST
IE 8.82 ± 0.02
eV
NIST
IE 8.87 ± 0.03
eV
NIST
IE Outlier eV
NIST
IE 9.15
eV
NIST
IE 9.00
eV
NIST
IE 9.18 ± 0.07
eV
NIST
log 10 WS0.22
Aq. Sol...
log Poct/wat 0.554
Crippen Calculated Property
McVol 84.450
ml/mol
McGowan Calculated Property
NFPA Fire 2
KDB
NFPA Health 2
KDB
Pc 4046.64
kPa
Joback Calculated Property
Inp [753.00; 804.00]
Inp 786.60
NIST
Inp 787.25
NIST
Inp 763.43
NIST
Inp 791.00
NIST
Inp 775.00
NIST
Inp 754.00
NIST
Inp 771.00
NIST
Inp 779.00
NIST
Inp 771.00
NIST
Inp 783.00
NIST
Inp 787.00
NIST
Inp 782.00
NIST
Inp 795.00
NIST
Inp 756.00
NIST
Inp 760.00
NIST
Inp 753.00
NIST
Inp 799.00
NIST
Inp 778.00
NIST
Inp 782.00
NIST
Inp 783.00
NIST
Inp 760.00
NIST
Inp 786.00
NIST
Inp 779.00
NIST
Inp 764.00
NIST
Inp 764.00
NIST
Inp 790.00
NIST
Inp 775.00
NIST
Inp 804.00
NIST
Inp 786.60
NIST
Inp 754.00
NIST
Inp 783.00
NIST
Inp 760.00
NIST
Inp 779.00
NIST
Inp 804.00
NIST
I [1167.00; 1230.00]
I 1196.00
NIST
I 1167.00
NIST
I 1200.00
NIST
I 1230.00
NIST
I 1196.00
NIST
I 1167.00
NIST
I 1230.00
NIST
Tboil [409.15; 413.60]
K
Tboil 413.60
K
Isobari...
Tboil 413.60
K
NIST
Tboil 411.00
K
NIST
Tboil 410.15 ± 1.50
K
NIST
Tboil 411.10
K
NIST
Tboil 410.15 ± 0.40
K
NIST
Tboil 410.65 ± 1.50
K
NIST
Tboil 410.70 ± 2.00
K
NIST
Tboil 409.15 ± 2.00
K
NIST
Tboil 409.15 ± 2.00
K
NIST
Tc 613.03
K
Joback Calculated Property
Tfus 249.95 ± 0.30
K
NIST
Ttriple 254.80 ± 0.20
K
NIST
Vc 0.328
m3 /kmol
Joback Calculated Property
Temperature Dependent Properties
Pressure Dependent Properties
Property
Value
Unit
Pressure (kPa)
Source
Tboilr 412.20
K
99.50
NIST
Correlations
Similar Compounds
Find more compounds similar to Acetylacetone .
Mixtures
Find more mixtures with Acetylacetone .
Sources
KDB Pure (Korean Thermophysical Properties Databank) KDB Vapor Pressure Data Crippen Method Viscosity Behavior of Some Oxygen Containing Compounds Determination and correlation of molar excess enthalpies of binary systems 2,4-pentanedione + (1-butanol, + 2-methyl-1-propanol, + 1-pentanol, + 1-heptane, + ethyl acetate, and + water) Liquid-liquid equilibrium for the ternary systems of water + acetyl acetone + propyl acetate at several temperatures Liquid-liquid equilibria, excess molar volume and deviations of the refractive indices at 298.15 K for mixtures of solvents used in themolybdenum extraction process (Vapor + liquid) equilibrium of the binary mixtures formed by acetonitrile with selected compounds at 95.5 kPa Bubble point measurements of binary mixtures formed by ethyl benzene with selected compounds at 95.35 kPa Concentration Dependence of Surface Tension for Very Dilute Aqueous Solutions of Organic Non-Electrolytes Liquid-Liquid Equilibrium of (Water + Pentane-2,4-dione + Ethyl Ethanoate) and (Water + Pentane-2,4-dione + Cyclohexane) at (298.15 and 313.15) K Liquid-Liquid Equilibrium, Solid-Liquid Equilibrium, Densities, and Refractivity of a Water, Chloroform, and Acetylacetone Mixture Critical Assessment of CO2 Solubility in Volatile Solvents at 298.15 K Excess Enthalpies of 2,4-Pentanedione + (Methanol, + Ethanol, + 1-Propanol, and + 2-Propanol) at T = (298.15, 313.15, and 328.15) K and p = (0.1 and 10.0) MPa Isobaric Vapor Liquid Equilibria for Binary Systems of Acetone + Isopropenyl Acetate, 2-Butanone + Isopropenyl Acetate, and Isopropenyl Acetate + Acetylacetone at 101.3 kPa Measurement of Infinite Dilution Activity Coefficients of Alcohols, Ketones, and Aromatic Hydrocarbons in 4-Methyl-N-butylpyridinium Tetrafluoroborate and 1-Butyl-3-methylimidazolium Hexafluorophosphate by Gas-Liquid Chromatography Isobaric Vapor-Liquid Equilibria for the Binary Systems of Acetic Acid + Isopropenyl Acetate, Acetic Acid + Acetylacetone, and Water + Acetylacetone Solubility of 1,1'-(Ethane-1,2-diyl)-bis(3-methylpyridinium) Dihexafluorophosphate in Different Solvents Solubilities of 1,1?-(Propane-1,3-diyl)-bis(3-methyl-1H-imidazolium-1-yl) Dihexafluorophosphate in Different Solvents Isobaric Vapor-Liquid Equilibria for (Acetic Acid + Cyclohexane) and (Cyclohexane + Acetylacetone) at a Pressure of 101.3 kPa and for (Acetic Acid + Acetylacetone) at a Pressure of 60.0 kPa Solubility of 1,1'-(Butane-1,4-diyl)-bis(3-methyl-1H-imidazolium-1-yl) Dihexafluorophosphate in Water, Acetophenone, Cyclohexanone, Acetylacetone, and 2-Butanone Solubility of 1,1'-(Butane-1,4-diyl)-bis(3-methylpyridinium) Dihexafluorophosphate in Different Solvents from (288.15 to 333.15) K Joback Method KDB Aqueous Solubility Prediction Method McGowan Method NIST Webbook The Yaws Handbook of Vapor Pressure
Note: Cheméo is only indexing the data, follow the source links to retrieve the latest data. The source is also providing more information like the publication year, authors and more.
Take the time to validate and double check the source of the data.
Outlier