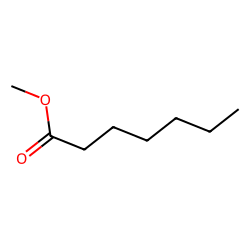
Contents
- Physical Properties
- Temperature Dependent Properties
- Datasets
- Correlations
- Similar Compounds
- Mixtures
- Sources
Physical Properties
| Property | Value | Unit | Source |
|---|---|---|---|
| ΔcH°liquid | -4867.40 ± 0.80 | kJ/mol | NIST |
| ΔfG° | -217.44 | kJ/mol | Joback Calculated Property |
| ΔfH°gas | -517.00 ± 2.00 | kJ/mol | NIST |
| ΔfH°liquid | -567.10 ± 0.92 | kJ/mol | NIST |
| ΔfusH° | 19.26 | kJ/mol | Joback Calculated Property |
| ΔvapH° | [49.70; 53.70] | kJ/mol |
|
| ΔvapH° | 53.20 ± 0.20 | kJ/mol | NIST |
| ΔvapH° | 51.80 ± 0.10 | kJ/mol | NIST |
| ΔvapH° | 53.40 | kJ/mol | NIST |
| ΔvapH° | 53.70 | kJ/mol | NIST |
| ΔvapH° | 53.50 | kJ/mol | NIST |
| ΔvapH° | 49.70 ± 0.50 | kJ/mol | NIST |
| ΔvapH° | 53.10 ± 0.40 | kJ/mol | NIST |
| ΔvapH° | 53.10 ± 0.10 | kJ/mol | NIST |
| ΔvapH° | 51.62 ± 0.48 | kJ/mol | NIST |
| ΔvapH° | 51.60 ± 0.50 | kJ/mol | NIST |
| ΔvapH° | 50.00 ± 1.00 | kJ/mol | NIST |
| ΔvapH° | 50.10 | kJ/mol | NIST |
| log10WS | -2.03 | Crippen Calculated Property | |
| logPoct/wat | 2.130 | Crippen Calculated Property | |
| McVol | 131.020 | ml/mol | McGowan Calculated Property |
| Pc | 2657.03 | kPa | Joback Calculated Property |
| Inp | [164.60; 1028.40] |
|
|
| Inp | 1023.90 | NIST | |
| Inp | 1023.30 | NIST | |
| Inp | 1022.90 | NIST | |
| Inp | 1005.00 | NIST | |
| Inp | 1012.00 | NIST | |
| Inp | 1002.00 | NIST | |
| Inp | 1006.00 | NIST | |
| Inp | 1011.00 | NIST | |
| Inp | 1007.00 | NIST | |
| Inp | 1026.00 | NIST | |
| Inp | 1008.60 | NIST | |
| Inp | 1008.10 | NIST | |
| Inp | 1008.00 | NIST | |
| Inp | 1027.70 | NIST | |
| Inp | 1028.40 | NIST | |
| Inp | 1021.00 | NIST | |
| Inp | 1005.00 | NIST | |
| Inp | 1004.00 | NIST | |
| Inp | 1007.00 | NIST | |
| Inp | 1007.00 | NIST | |
| Inp | 1006.00 | NIST | |
| Inp | 1026.00 | NIST | |
| Inp | 1013.00 | NIST | |
| Inp | 1013.00 | NIST | |
| Inp | 1005.00 | NIST | |
| Inp | 1009.00 | NIST | |
| Inp | 1010.00 | NIST | |
| Inp | 1006.00 | NIST | |
| Inp | 1006.00 | NIST | |
| Inp | 1006.00 | NIST | |
| Inp | 1025.00 | NIST | |
| Inp | 1005.00 | NIST | |
| Inp | 1025.00 | NIST | |
| Inp | 1021.00 | NIST | |
| Inp | 1006.00 | NIST | |
| Inp | 1006.00 | NIST | |
| Inp | 1014.00 | NIST | |
| Inp | 1005.00 | NIST | |
| Inp | 1006.00 | NIST | |
| Inp | 1006.00 | NIST | |
| Inp | 1008.00 | NIST | |
| Inp | Outlier 164.60 | NIST | |
| Inp | 1006.00 | NIST | |
| Inp | Outlier 164.60 | NIST | |
| Inp | 1025.00 | NIST | |
| Inp | 1008.00 | NIST | |
| Inp | 1009.00 | NIST | |
| Inp | 1023.90 | NIST | |
| Inp | 1002.00 | NIST | |
| I | [1241.00; 1327.00] |
|
|
| I | Outlier 1241.00 | NIST | |
| I | Outlier 1327.00 | NIST | |
| I | 1281.00 | NIST | |
| I | 1291.00 | NIST | |
| I | 1301.00 | NIST | |
| I | 1292.00 | NIST | |
| I | 1299.00 | NIST | |
| I | 1291.00 | NIST | |
| I | 1282.00 | NIST | |
| I | 1288.00 | NIST | |
| I | 1296.00 | NIST | |
| I | 1284.00 | NIST | |
| I | 1273.00 | NIST | |
| I | 1279.00 | NIST | |
| I | 1273.00 | NIST | |
| I | 1279.00 | NIST | |
| I | 1279.00 | NIST | |
| I | 1296.00 | NIST | |
| I | 1276.00 | NIST | |
| I | 1273.00 | NIST | |
| I | 1302.00 | NIST | |
| I | 1281.00 | NIST | |
| I | 1291.00 | NIST | |
| I | 1282.00 | NIST | |
| I | 1296.00 | NIST | |
| I | 1288.00 | NIST | |
| Tboil | [445.20; 446.95] | K |
|
| Tboil | 445.20 | K | NIST |
| Tboil | 446.95 ± 0.30 | K | NIST |
| Tboil | 445.30 ± 2.00 | K | NIST |
| Tc | 633.80 | K | Joback Calculated Property |
| Tfus | [217.40; 217.40] | K |
|
| Tfus | 217.40 ± 0.05 | K | NIST |
| Tfus | 217.40 ± 0.50 | K | NIST |
| Vc | 0.507 | m3/kmol | Joback Calculated Property |
Temperature Dependent Properties
| Property | Value | Unit | Temperature (K) | Source |
|---|---|---|---|---|
| Cp,gas | [279.70; 346.77] | J/mol×K | [458.73; 633.80] | |
|
T(K) Ideal gas heat capacity (J/mol×K) 280 290 300 310 320 330 340 350 500 550 600 | ||||
| Cp,gas | 279.70 | J/mol×K | 458.73 | Joback Calculated Property |
| Cp,gas | 291.94 | J/mol×K | 487.91 | Joback Calculated Property |
| Cp,gas | 303.75 | J/mol×K | 517.09 | Joback Calculated Property |
| Cp,gas | 315.14 | J/mol×K | 546.26 | Joback Calculated Property |
| Cp,gas | 326.10 | J/mol×K | 575.44 | Joback Calculated Property |
| Cp,gas | 336.64 | J/mol×K | 604.62 | Joback Calculated Property |
| Cp,gas | 346.77 | J/mol×K | 633.80 | Joback Calculated Property |
| Cp,liquid | [285.10; 316.90] | J/mol×K | [298.15; 373.15] | |
|
T(K) Liquid phase heat capacity (J/mol×K) 285 290 295 300 305 310 315 320 300 320 340 360 | ||||
| Cp,liquid | 285.10 | J/mol×K | 298.15 | NIST |
| Cp,liquid | 289.20 | J/mol×K | 303.15 | Heat ca... |
| Cp,liquid | 292.50 | J/mol×K | 313.15 | Heat ca... |
| Cp,liquid | 296.20 | J/mol×K | 323.15 | Heat ca... |
| Cp,liquid | 300.10 | J/mol×K | 333.15 | Heat ca... |
| Cp,liquid | 304.30 | J/mol×K | 343.15 | Heat ca... |
| Cp,liquid | 308.60 | J/mol×K | 353.15 | Heat ca... |
| Cp,liquid | 312.80 | J/mol×K | 363.15 | Heat ca... |
| Cp,liquid | 316.90 | J/mol×K | 373.15 | Heat ca... |
| η | [0.0008230; 0.0013200] | Pa×s | [283.15; 313.15] | |
|
T(K) Dynamic viscosity (Pa×s) 8.00e-4 9.00e-4 1.00e-3 1.10e-3 1.20e-3 1.30e-3 290 300 310 | ||||
| η | 0.0013200 | Pa×s | 283.15 | A Study... |
| η | 0.0011120 | Pa×s | 293.15 | A Study... |
| η | 0.0010230 | Pa×s | 298.15 | A Study... |
| η | 0.0009500 | Pa×s | 303.15 | A Study... |
| η | 0.0008230 | Pa×s | 313.15 | A Study... |
| ΔvapH | [46.30; 50.20] | kJ/mol | [326.00; 432.50] | |
| ΔvapH | 50.20 ± 0.10 | kJ/mol | 326.00 | NIST |
| ΔvapH | 49.10 | kJ/mol | 350.00 | NIST |
| ΔvapH | 49.00 | kJ/mol | 367.00 | NIST |
| ΔvapH | 46.30 | kJ/mol | 432.50 | NIST |
| Pvap | [0.03; 0.47] | kPa | [278.10; 313.20] | |
|
T(K) Vapor pressure (kPa) 0 0.1 0.2 0.3 0.4 0.5 280 290 300 310 | ||||
| Pvap | 0.03 | kPa | 278.10 | Transpi... |
| Pvap | 0.05 | kPa | 283.50 | Transpi... |
| Pvap | 0.07 | kPa | 286.40 | Transpi... |
| Pvap | 0.08 | kPa | 288.30 | Transpi... |
| Pvap | 0.09 | kPa | 290.30 | Transpi... |
| Pvap | 0.12 | kPa | 293.20 | Transpi... |
| Pvap | 0.14 | kPa | 296.20 | Transpi... |
| Pvap | 0.16 | kPa | 298.20 | Transpi... |
| Pvap | 0.19 | kPa | 300.30 | Transpi... |
| Pvap | 0.23 | kPa | 303.10 | Transpi... |
| Pvap | 0.28 | kPa | 306.10 | Transpi... |
| Pvap | 0.33 | kPa | 308.20 | Transpi... |
| Pvap | 0.38 | kPa | 310.20 | Transpi... |
| Pvap | 0.47 | kPa | 313.20 | Transpi... |
| n0 | [1.38276; 1.41865] | [278.15; 358.15] | ||
|
T(K) Refractive Index 1.38 1.39 1.4 1.41 1.42 300 350 | ||||
| n0 | 1.41865 | 278.15 | Thermop... | |
| n0 | 1.41643 | 283.15 | Thermop... | |
| n0 | 1.41421 | 288.15 | Thermop... | |
| n0 | 1.41199 | 293.15 | Thermop... | |
| n0 | 1.40945 | 298.15 | Densiti... | |
| n0 | 1.40976 | 298.15 | Thermop... | |
| n0 | 1.40945 | 298.15 | Isobari... | |
| n0 | 1.40753 | 303.15 | Thermop... | |
| n0 | 1.40530 | 308.15 | Thermop... | |
| n0 | 1.40301 | 313.15 | Thermop... | |
| n0 | 1.40070 | 318.15 | Thermop... | |
| n0 | 1.39841 | 323.15 | Thermop... | |
| n0 | 1.39614 | 328.15 | Thermop... | |
| n0 | 1.39389 | 333.15 | Thermop... | |
| n0 | 1.39167 | 338.15 | Thermop... | |
| n0 | 1.38946 | 343.15 | Thermop... | |
| n0 | 1.38724 | 348.15 | Thermop... | |
| n0 | 1.38501 | 353.15 | Thermop... | |
| n0 | 1.38276 | 358.15 | Thermop... | |
| ρl | 875.61 | kg/m3 | 298.15 | Densiti... |
Datasets
Viscosity, Pa*s
| Fixed | Measured | |
|---|---|---|
| Temperature, K - Liquid | Pressure, kPa - Liquid | Viscosity, Pa*s - Liquid |
| 294.20 | 107.00 | 0.0011 |
| 294.13 | 4967.00 | 0.0012 |
| 293.85 | 9970.00 | 0.0012 |
| 293.58 | 14994.00 | 0.0013 |
| 292.87 | 19994.00 | 0.0014 |
| 292.56 | 25040.00 | 0.0014 |
| 292.04 | 30028.00 | 0.0015 |
| 303.15 | 102.00 | 0.0009 |
| 303.12 | 5011.00 | 0.0010 |
| 303.08 | 10026.00 | 0.0010 |
| 303.05 | 14993.00 | 0.0011 |
| 303.02 | 19985.00 | 0.0011 |
| 302.93 | 25017.00 | 0.0012 |
| 302.83 | 29961.00 | 0.0012 |
| 312.77 | 103.00 | 0.0008 |
| 312.78 | 5010.00 | 0.0009 |
| 312.65 | 10009.00 | 0.0009 |
| 312.66 | 15001.00 | 0.0010 |
| 312.62 | 20047.00 | 0.0010 |
| 312.57 | 25001.00 | 0.0010 |
| 312.49 | 29986.00 | 0.0011 |
| 322.33 | 96.00 | 0.0007 |
| 322.26 | 5004.00 | 0.0008 |
| 322.23 | 10018.00 | 0.0008 |
| 322.21 | 15004.00 | 0.0008 |
| 322.17 | 19955.00 | 0.0009 |
| 322.23 | 25027.00 | 0.0009 |
| 322.09 | 29925.00 | 0.0010 |
| 331.85 | 99.00 | 0.0006 |
| 331.78 | 5007.00 | 0.0007 |
| 331.78 | 9972.00 | 0.0007 |
| 331.74 | 15035.00 | 0.0008 |
| 331.67 | 19865.00 | 0.0008 |
| 331.68 | 25041.00 | 0.0008 |
| 331.56 | 30001.00 | 0.0009 |
| 341.67 | 96.00 | 0.0006 |
| 341.72 | 5062.00 | 0.0006 |
| 341.68 | 10141.00 | 0.0006 |
| 341.48 | 14961.00 | 0.0007 |
| 341.81 | 20048.00 | 0.0007 |
| 341.80 | 25032.00 | 0.0008 |
| 341.73 | 29980.00 | 0.0008 |
| 351.27 | 10077.00 | 0.0006 |
| 351.26 | 14966.00 | 0.0006 |
| 351.31 | 19977.00 | 0.0006 |
| 351.49 | 24997.00 | 0.0007 |
| 351.46 | 30010.00 | 0.0007 |
| 361.37 | 20002.00 | 0.0006 |
| 361.47 | 25022.00 | 0.0006 |
| 361.24 | 29993.00 | 0.0006 |
| Reference | ||
Mass density, kg/m3
| Fixed | Measured | |
|---|---|---|
| Temperature, K - Liquid | Pressure, kPa - Liquid | Mass density, kg/m3 - Liquid |
| 293.15 | 100.00 | 880.16 |
| 293.15 | 1000.00 | 880.29 |
| 293.15 | 3000.00 | 881.98 |
| 293.15 | 5000.00 | 883.19 |
| 293.15 | 10000.00 | 886.99 |
| 293.15 | 15000.00 | 890.49 |
| 293.15 | 20000.00 | 893.86 |
| 293.15 | 25000.00 | 897.18 |
| 293.15 | 30000.00 | 900.36 |
| 293.15 | 35000.00 | 903.34 |
| 293.15 | 40000.00 | 906.22 |
| 293.15 | 50000.00 | 912.14 |
| 293.15 | 60000.00 | 917.16 |
| 303.15 | 100.00 | 870.66 |
| 303.15 | 1000.00 | 871.46 |
| 303.15 | 3000.00 | 873.07 |
| 303.15 | 5000.00 | 874.66 |
| 303.15 | 10000.00 | 878.5 |
| 303.15 | 15000.00 | 882.18 |
| 303.15 | 20000.00 | 885.64 |
| 303.15 | 25000.00 | 889.08 |
| 303.15 | 30000.00 | 892.3 |
| 303.15 | 35000.00 | 895.46 |
| 303.15 | 40000.00 | 898.49 |
| 303.15 | 50000.00 | 904.43 |
| 303.15 | 60000.00 | 909.95 |
| 313.15 | 100.00 | 861.82 |
| 313.15 | 1000.00 | 862.16 |
| 313.15 | 3000.00 | 863.9 |
| 313.15 | 5000.00 | 865.62 |
| 313.15 | 10000.00 | 869.71 |
| 313.15 | 15000.00 | 873.55 |
| 313.15 | 20000.00 | 877.29 |
| 313.15 | 25000.00 | 880.89 |
| 313.15 | 30000.00 | 884.32 |
| 313.15 | 35000.00 | 887.66 |
| 313.15 | 40000.00 | 890.73 |
| 313.15 | 50000.00 | 896.98 |
| 313.15 | 60000.00 | 902.59 |
| 323.15 | 100.00 | 852.25 |
| 323.15 | 1000.00 | 853.12 |
| 323.15 | 3000.00 | 854.89 |
| 323.15 | 5000.00 | 856.64 |
| 323.15 | 10000.00 | 860.97 |
| 323.15 | 15000.00 | 865.07 |
| 323.15 | 20000.00 | 869.08 |
| 323.15 | 25000.00 | 872.69 |
| 323.15 | 30000.00 | 876.31 |
| 323.15 | 35000.00 | 879.74 |
| 323.15 | 40000.00 | 883.15 |
| 323.15 | 50000.00 | 889.53 |
| 323.15 | 60000.00 | 895.63 |
| 333.15 | 100.00 | 843.03 |
| 333.15 | 1000.00 | 843.81 |
| 333.15 | 3000.00 | 845.84 |
| 333.15 | 5000.00 | 847.68 |
| 333.15 | 10000.00 | 852.16 |
| 333.15 | 15000.00 | 856.43 |
| 333.15 | 20000.00 | 860.63 |
| 333.15 | 25000.00 | 864.64 |
| 333.15 | 30000.00 | 868.31 |
| 333.15 | 35000.00 | 871.91 |
| 333.15 | 40000.00 | 875.61 |
| 333.15 | 50000.00 | 882.2 |
| 333.15 | 60000.00 | 888.39 |
| 343.15 | 100.00 | 833.48 |
| 343.15 | 1000.00 | 834.64 |
| 343.15 | 3000.00 | 836.7 |
| 343.15 | 5000.00 | 838.79 |
| 343.15 | 10000.00 | 843.47 |
| 343.15 | 15000.00 | 848.02 |
| 343.15 | 20000.00 | 852.59 |
| 343.15 | 25000.00 | 856.55 |
| 343.15 | 30000.00 | 860.57 |
| 343.15 | 35000.00 | 864.43 |
| 343.15 | 40000.00 | 867.96 |
| 343.15 | 50000.00 | 875.0 |
| 343.15 | 60000.00 | 881.19 |
| 353.15 | 100.00 | 824.35 |
| 353.15 | 1000.00 | 825.27 |
| 353.15 | 3000.00 | 827.61 |
| 353.15 | 5000.00 | 829.66 |
| 353.15 | 10000.00 | 834.76 |
| 353.15 | 15000.00 | 839.63 |
| 353.15 | 20000.00 | 844.11 |
| 353.15 | 25000.00 | 848.48 |
| 353.15 | 30000.00 | 852.65 |
| 353.15 | 35000.00 | 856.65 |
| 353.15 | 40000.00 | 860.37 |
| 353.15 | 50000.00 | 867.57 |
| 353.15 | 60000.00 | 874.32 |
| 363.15 | 100.00 | 815.19 |
| 363.15 | 1000.00 | 816.36 |
| 363.15 | 3000.00 | 818.75 |
| 363.15 | 5000.00 | 821.0 |
| 363.15 | 10000.00 | 826.14 |
| 363.15 | 15000.00 | 831.57 |
| 363.15 | 20000.00 | 836.11 |
| 363.15 | 25000.00 | 840.56 |
| 363.15 | 30000.00 | 845.01 |
| 363.15 | 35000.00 | 849.23 |
| 363.15 | 40000.00 | 853.33 |
| 363.15 | 50000.00 | 860.82 |
| 363.15 | 60000.00 | 867.91 |
| Reference | ||
Correlations
| Property | Value | Unit | Temperature (K) | Source |
|---|---|---|---|---|
| Pvap | [1.33; 202.64] | kPa | [334.72; 471.89] |
The Yaw...
|
| Equation | ln(Pvp) = A + B/(T + C) | |||
| Coefficient A | 1.51732e+01 | |||
| Coefficient B | -4.00813e+03 | |||
| Coefficient C | -6.54580e+01 | |||
| Temperature range, min. | 334.72 | |||
| Temperature range, max. | 471.89 | |||
|
T(K) Vapor pressure (kPa) 0 50 100 150 200 350 400 450 | ||||
| Pvap | 1.33 | kPa | 334.72 | Calculated Property |
| Pvap | 2.96 | kPa | 349.96 | Calculated Property |
| Pvap | 6.06 | kPa | 365.20 | Calculated Property |
| Pvap | 11.57 | kPa | 380.44 | Calculated Property |
| Pvap | 20.82 | kPa | 395.68 | Calculated Property |
| Pvap | 35.56 | kPa | 410.93 | Calculated Property |
| Pvap | 58.05 | kPa | 426.17 | Calculated Property |
| Pvap | 91.09 | kPa | 441.41 | Calculated Property |
| Pvap | 138.00 | kPa | 456.65 | Calculated Property |
| Pvap | 202.64 | kPa | 471.89 | Calculated Property |
Similar Compounds
Find more compounds similar to Heptanoic acid, methyl ester.
Mixtures
- Toluene + Heptanoic acid, methyl ester + Acetic acid, methyl ester
- Heptanoic acid, methyl ester + Water
- Heptanoic acid, methyl ester + 1-Hexanol
- Octane + Heptanoic acid, methyl ester + 1-Hexanol
- Benzene + Heptanoic acid, methyl ester
- Carbon dioxide + Heptanoic acid, methyl ester
Sources
- Crippen Method
- Crippen Method
- Transpiration method: Vapor pressures and enthalpies of vaporization of some low-boiling esters
- High-pressure liquid densities of fatty acid methyl esters: Measurement and prediction with the PC-SAFT equation of state
- Densities, speeds of sound, and refractive indices of the ternary mixtures (toluene + methyl acetate + butyl acetate) and (toluene + methyl acetate + methyl heptanoate) at 298.15 K
- Densities and interfacial tensions for fatty acid methyl esters (from methyl formate to methyl heptanoate) + water demixed mixtures at atmospheric pressure conditions
- Solubilities of CO2 capture absorbents methyl benzoate, ethyl hexanoate and methyl heptanoate
- Below the room temperature measurements of solubilities in ester absorbents for CO2 capture
- Heat capacities and thermal diffusivities of some n-alkanoic acid methyl esters
- Thermophysical properties of fatty acid methyl and ethyl esters
- Liquid viscosities for methyl hexanoate, methyl heptanoate, methyl caprylate, and methyl nonanoate at high pressures
- A Study on Properties Derived from Densities and Viscosities for the Ternary Systems (Methyl Pentanoate or Methyl Heptanoate) + n-Octane + 1-Hexanol and their Binary Subsystems at Various Temperatures.
- Thermodynamic Properties of Mixtures Containing Ionic Liquids. 8. Activity Coefficients at Infinite Dilution of Hydrocarbons, Alcohols, Esters, and Aldehydes in 1-Hexyl-3-methylimidazolium Bis(trifluoromethylsulfonyl) Imide Using Gas-Liquid Chromatography
- Thermodynamic Properties of Mixtures Containing Ionic Liquids. 9. Activity Coefficients at Infinite Dilution of Hydrocarbons, Alcohols, Esters, and Aldehydes in Trimethyl-butylammonium Bis(trifluoromethylsulfonyl) Imide Using Gas-Liquid Chromatography and Static Method
- Experimental Study of Thermodynamic Properties of Mixtures Containing Ionic Liquid 1-Ethyl-3-methylimidazolium Ethyl Sulfate Using Gas-Liquid Chromatography and Transpiration Method
- Isobaric Vapor-Liquid Equilibria for the Binary Systems Benzene + Methyl Ethanoate, Benzene + Butyl Ethanoate, and Benzene + Methyl Heptanoate at 101.31kPa
- Joback Method
- McGowan Method
- NIST Webbook
- The Yaws Handbook of Vapor Pressure
Note: Cheméo is only indexing the data, follow the source links to retrieve the latest data. The source is also providing more information like the publication year, authors and more.
Take the time to validate and double check the source of the data.
Outlier This icon means
that the value is more than 2 standard deviations away from the
property mean.