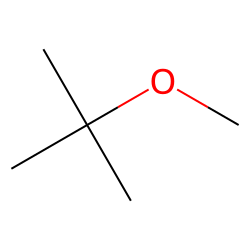
Physical Properties
| Property | Value | Unit | Source |
|---|---|---|---|
| ω | 0.2690 | KDB | |
| PAff | 841.60 | kJ/mol | NIST |
| BasG | 812.40 | kJ/mol | NIST |
| ΔcH°liquid | [-3368.97; -3359.70] | kJ/mol |
|
| ΔcH°liquid | -3368.97 | kJ/mol | NIST |
| ΔcH°liquid | -3359.70 ± 6.50 | kJ/mol | NIST |
| μ | 1.20 | debye | KDB |
| η | 0.0003610 | Pa×s | Densiti... |
| ΔfG° | -125.50 | kJ/mol | KDB |
| ΔfH°gas | [-293.10; -282.20] | kJ/mol |
|
| ΔfH°gas | -293.10 | kJ/mol | KDB |
| ΔfH°gas | -285.00 | kJ/mol | NIST |
| ΔfH°gas | -283.20 ± 1.30 | kJ/mol | NIST |
| ΔfH°gas | -282.20 ± 1.90 | kJ/mol | NIST |
| ΔfH°liquid | [-322.90; -313.60] | kJ/mol |
|
| ΔfH°liquid | -315.40 | kJ/mol | NIST |
| ΔfH°liquid | -313.60 ± 1.30 | kJ/mol | NIST |
| ΔfH°liquid | -322.90 ± 5.00 | kJ/mol | NIST |
| ΔfusH° | 2.48 | kJ/mol | Joback Calculated Property |
| ΔvapH° | 27.84 | kJ/mol | Joback Calculated Property |
| IE | [9.24; 9.48] | eV |
|
| IE | 9.24 | eV | NIST |
| IE | 9.48 | eV | NIST |
| IE | 9.41 | eV | NIST |
| log10WS | [-0.24; -0.24] |
|
|
| log10WS | -0.24 | Aq. Sol... | |
| log10WS | -0.24 | Estimat... | |
| logPoct/wat | 1.431 | Crippen Calculated Property | |
| McVol | 87.180 | ml/mol | McGowan Calculated Property |
| Pc | [3397.00; 3430.00] | kPa |
|
| Pc | 3430.00 | kPa | KDB |
| Pc | 3397.00 ± 8.00 | kPa | NIST |
| Pc | 3430.00 ± 10.00 | kPa | NIST |
| Inp | [544.00; 570.12] |
|
|
| Inp | 563.70 | NIST | |
| Inp | 562.70 | NIST | |
| Inp | 562.00 | NIST | |
| Inp | Outlier 544.00 | NIST | |
| Inp | 549.00 | NIST | |
| Inp | 570.12 | NIST | |
| Inp | 568.00 | NIST | |
| Inp | 566.00 | NIST | |
| Inp | 566.00 | NIST | |
| Inp | 567.00 | NIST | |
| Inp | 554.50 | NIST | |
| Inp | 558.00 | NIST | |
| Inp | 563.00 | NIST | |
| Inp | 570.00 | NIST | |
| Inp | 560.00 | NIST | |
| Inp | 556.00 | NIST | |
| Inp | 556.00 | NIST | |
| Inp | 556.00 | NIST | |
| Inp | 560.00 | NIST | |
| Inp | 568.00 | NIST | |
| Inp | 563.00 | NIST | |
| Inp | 556.00 | NIST | |
| I | [666.00; 688.00] |
|
|
| I | 666.00 | NIST | |
| I | 688.00 | NIST | |
| S°gas | 357.80 | J/mol×K | NIST |
| S°liquid | 265.30 | J/mol×K | NIST |
| Tboil | [328.25; 328.30] | K |
|
| Tboil | 328.30 | K | KDB |
| Tboil | 328.25 | K | Vapor-L... |
| Tc | [496.40; 497.10] | K |
|
| Tc | 497.10 | K | KDB |
| Tc | 497.00 | K | Measure... |
| Tc | 496.40 ± 0.30 | K | NIST |
| Tc | 497.10 | K | NIST |
| Tc | 497.10 ± 0.20 | K | NIST |
| Tfus | [164.25; 164.50] | K |
|
| Tfus | 164.50 | K | KDB |
| Tfus | 164.25 | K | Aq. Sol... |
| Tfus | 164.50 ± 0.20 | K | NIST |
| Ttriple | 164.56 ± 0.07 | K | NIST |
| Vc | 0.323 | m3/kmol | KDB |
| Zc | 0.2676350 | KDB |
Temperature Dependent Properties
| Property | Value | Unit | Temperature (K) | Source |
|---|---|---|---|---|
| Cp,gas | [147.83; 203.44] | J/mol×K | [332.99; 508.60] | |
|
T(K) Ideal gas heat capacity (J/mol×K) 150 160 170 180 190 200 350 400 450 500 | ||||
| Cp,gas | 147.83 | J/mol×K | 332.99 | Joback Calculated Property |
| Cp,gas | 158.08 | J/mol×K | 362.26 | Joback Calculated Property |
| Cp,gas | 167.93 | J/mol×K | 391.53 | Joback Calculated Property |
| Cp,gas | 177.38 | J/mol×K | 420.79 | Joback Calculated Property |
| Cp,gas | 186.44 | J/mol×K | 450.06 | Joback Calculated Property |
| Cp,gas | 195.13 | J/mol×K | 479.33 | Joback Calculated Property |
| Cp,gas | 203.44 | J/mol×K | 508.60 | Joback Calculated Property |
| Cp,liquid | [187.50; 188.00] | J/mol×K | [298.00; 298.15] | |
| Cp,liquid | 188.00 | J/mol×K | 298.00 | NIST |
| Cp,liquid | 187.50 | J/mol×K | 298.15 | NIST |
| Cp,liquid | 187.80 | J/mol×K | 298.15 | NIST |
| η | [0.0002780; 0.0004430] | Pa×s | [273.15; 313.15] | |
|
T(K) Dynamic viscosity (Pa×s) 3.00e-4 3.50e-4 4.00e-4 4.50e-4 280 290 300 310 | ||||
| η | 0.0003900 | Pa×s | 273.15 | Density... |
| η | 0.0004430 | Pa×s | 273.15 | Densiti... |
| η | 0.0004170 | Pa×s | 278.15 | Densiti... |
| η | 0.0003920 | Pa×s | 283.15 | Densiti... |
| η | 0.0003600 | Pa×s | 283.15 | Density... |
| η | 0.0003897 | Pa×s | 283.15 | Experim... |
| η | 0.0003640 | Pa×s | 288.15 | Experim... |
| η | 0.0003700 | Pa×s | 288.15 | Densiti... |
| η | 0.0003482 | Pa×s | 293.15 | Experim... |
| η | 0.0003330 | Pa×s | 293.15 | Density... |
| η | 0.0003327 | Pa×s | 298.15 | Experim... |
| η | 0.0003190 | Pa×s | 298.15 | Density... |
| η | 0.0003040 | Pa×s | 303.15 | Density... |
| η | 0.0003152 | Pa×s | 303.15 | Experim... |
| η | 0.0002994 | Pa×s | 308.15 | Experim... |
| η | 0.0002846 | Pa×s | 313.15 | Experim... |
| η | 0.0002780 | Pa×s | 313.15 | Density... |
| ΔfusH | [7.60; 7.60] | kJ/mol | [164.56; 164.60] | |
| ΔfusH | 7.60 | kJ/mol | 164.56 | NIST |
| ΔfusH | 7.60 | kJ/mol | 164.60 | NIST |
| ΔfusH | 7.60 | kJ/mol | 164.60 | NIST |
| ΔvapH | [27.90; 31.20] | kJ/mol | [306.50; 355.50] | |
|
T(K) Enthalpy of vaporization at a given temperature (kJ/mol) 28 28.5 29 29.5 30 30.5 31 31.5 320 340 | ||||
| ΔvapH | 30.40 | kJ/mol | 306.50 | NIST |
| ΔvapH | 30.00 | kJ/mol | 310.00 | NIST |
| ΔvapH | 29.90 | kJ/mol | 314.00 | NIST |
| ΔvapH | 30.20 | kJ/mol | 319.00 | NIST |
| ΔvapH | 27.90 | kJ/mol | 328.00 | NIST |
| ΔvapH | 27.94 | kJ/mol | 328.30 | NIST |
| ΔvapH | 29.60 | kJ/mol | 340.00 | NIST |
| ΔvapH | 31.20 | kJ/mol | 355.50 | NIST |
| ν | [0.0000004; 0.0000005] | m2/s | [283.15; 313.15] | |
| ν | 0.0000005 | m2/s | 283.15 | Kinemat... |
| ν | 0.0000005 | m2/s | 298.15 | Kinemat... |
| ν | 0.0000004 | m2/s | 313.15 | Kinemat... |
| Pvap | [6.24; 221.79] | kPa | [263.15; 354.82] | |
|
T(K) Vapor pressure (kPa) 0 50 100 150 200 280 300 320 340 | ||||
| Pvap | 6.24 | kPa | 263.15 | Isother... |
| Pvap | 6.25 | kPa | 263.15 | Thermod... |
| Pvap | 6.40 | kPa | 263.53 | Isother... |
| Pvap | 10.67 | kPa | 273.15 | Isother... |
| Pvap | 10.64 | kPa | 273.15 | Thermod... |
| Pvap | 10.80 | kPa | 273.34 | Isother... |
| Pvap | 11.59 | kPa | 274.90 | Isother... |
| Pvap | 11.59 | kPa | 274.91 | Isother... |
| Pvap | 17.41 | kPa | 283.15 | Isother... |
| Pvap | 17.31 | kPa | 283.15 | Thermod... |
| Pvap | 17.60 | kPa | 283.27 | Isother... |
| Pvap | 17.79 | kPa | 283.60 | A milli... |
| Pvap | 18.55 | kPa | 284.82 | Isother... |
| Pvap | 22.07 | kPa | 288.60 | A milli... |
| Pvap | 27.06 | kPa | 293.15 | Thermod... |
| Pvap | 27.25 | kPa | 293.15 | Isother... |
| Pvap | 27.40 | kPa | 293.25 | Isother... |
| Pvap | 27.43 | kPa | 293.50 | A milli... |
| Pvap | 28.78 | kPa | 294.80 | Isother... |
| Pvap | 28.78 | kPa | 294.80 | Isother... |
| Pvap | 28.78 | kPa | 294.81 | Isother... |
| Pvap | 33.39 | kPa | 298.15 | Thermod... |
| Pvap | 33.72 | kPa | 298.40 | A milli... |
| Pvap | 41.16 | kPa | 303.15 | Isother... |
| Pvap | 40.86 | kPa | 303.15 | Thermod... |
| Pvap | 41.20 | kPa | 303.22 | Isother... |
| Pvap | 41.10 | kPa | 303.30 | A milli... |
| Pvap | 43.26 | kPa | 304.79 | Isother... |
| Pvap | 43.24 | kPa | 304.79 | Isother... |
| Pvap | 49.80 | kPa | 308.20 | A milli... |
| Pvap | 59.90 | kPa | 313.10 | A milli... |
| Pvap | 60.22 | kPa | 313.15 | Isother... |
| Pvap | 59.81 | kPa | 313.15 | Thermod... |
| Pvap | 62.40 | kPa | 313.20 | Isother... |
| Pvap | 60.30 | kPa | 313.24 | Isother... |
| Pvap | 62.95 | kPa | 314.75 | Isother... |
| Pvap | 62.97 | kPa | 314.76 | Isother... |
| Pvap | 62.97 | kPa | 314.76 | Isother... |
| Pvap | 71.61 | kPa | 318.10 | A milli... |
| Pvap | 85.01 | kPa | 323.00 | A milli... |
| Pvap | 85.15 | kPa | 323.15 | Thermod... |
| Pvap | 85.65 | kPa | 323.15 | Isother... |
| Pvap | 85.80 | kPa | 323.21 | Isother... |
| Pvap | 89.66 | kPa | 324.90 | Isother... |
| Pvap | 89.69 | kPa | 324.90 | Isother... |
| Pvap | 100.34 | kPa | 327.90 | A milli... |
| Pvap | 117.70 | kPa | 332.90 | A milli... |
| Pvap | 118.27 | kPa | 333.15 | Thermod... |
| Pvap | 118.78 | kPa | 333.15 | Isother... |
| Pvap | 120.40 | kPa | 333.15 | Thermod... |
| Pvap | 119.20 | kPa | 333.19 | Isother... |
| Pvap | 124.02 | kPa | 334.85 | Isother... |
| Pvap | 137.39 | kPa | 337.80 | A milli... |
| Pvap | 160.66 | kPa | 343.15 | Thermod... |
| Pvap | 167.14 | kPa | 344.84 | Isother... |
| Pvap | 167.57 | kPa | 344.85 | Isother... |
| Pvap | 221.79 | kPa | 354.82 | Isother... |
| Pvap | 221.39 | kPa | 354.82 | Isother... |
| n0 | [1.36594; 1.36980] | [293.15; 298.20] | ||
|
T(K) Refractive Index 1.37 1.37 1.37 1.37 1.37 294 296 298 | ||||
| n0 | 1.36900 | 293.15 | (Vapor ... | |
| n0 | 1.36890 | 293.15 | A novel... | |
| n0 | 1.36980 | 293.15 | Vapor-L... | |
| n0 | 1.36766 | 298.15 | Phase e... | |
| n0 | 1.36648 | 298.15 | Volumet... | |
| n0 | 1.36648 | 298.15 | Volumet... | |
| n0 | 1.36610 | 298.15 | sotherm... | |
| n0 | 1.36594 | 298.15 | Quatern... | |
| n0 | 1.36628 | 298.15 | Isobari... | |
| n0 | 1.36600 | 298.15 | Solubil... | |
| n0 | 1.36764 | 298.15 | Atmosph... | |
| n0 | 1.36766 | 298.15 | Vapor-L... | |
| n0 | 1.36632 | 298.20 | Vapor-L... | |
| ρl | [713.96; 761.34] | kg/m3 | [273.15; 318.15] | |
|
T(K) Liquid Density (kg/m3) 710 720 730 740 750 760 280 290 300 310 | ||||
| ρl | 761.30 | kg/m3 | 273.15 | Physico... |
| ρl | 761.34 | kg/m3 | 273.15 | Densiti... |
| ρl | 756.14 | kg/m3 | 278.15 | Densiti... |
| ρl | 751.02 | kg/m3 | 283.15 | Densiti... |
| ρl | 745.90 | kg/m3 | 288.15 | Physico... |
| ρl | 745.85 | kg/m3 | 288.15 | Densiti... |
| ρl | 740.65 | kg/m3 | 293.15 | Densiti... |
| ρl | 739.80 | kg/m3 | 293.15 | Measure... |
| ρl | 739.45 | kg/m3 | 293.20 | Liquid-... |
| ρl | 735.40 | kg/m3 | 298.15 | Phase e... |
| ρl | 734.10 | kg/m3 | 298.15 | Solubil... |
| ρl | 735.69 | kg/m3 | 298.15 | Excess ... |
| ρl | 735.36 | kg/m3 | 298.15 | Densiti... |
| ρl | 735.69 | kg/m3 | 298.15 | Excess ... |
| ρl | 735.00 | kg/m3 | 298.15 | Physico... |
| ρl | 735.99 | kg/m3 | 298.15 | Excess ... |
| ρl | 735.35 | kg/m3 | 298.15 | Mixing ... |
| ρl | 735.48 | kg/m3 | 298.15 | Excess ... |
| ρl | 740.60 | kg/m3 | 298.15 | Vapor-L... |
| ρl | 735.21 | kg/m3 | 298.15 | Excess ... |
| ρl | 735.99 | kg/m3 | 298.15 | Excess ... |
| ρl | 735.99 | kg/m3 | 298.15 | Excess ... |
| ρl | 735.40 | kg/m3 | 298.15 | Densiti... |
| ρl | 737.80 | kg/m3 | 298.20 | Phase e... |
| ρl | 730.10 | kg/m3 | 303.15 | Densiti... |
| ρl | 724.60 | kg/m3 | 308.15 | Physico... |
| ρl | 724.85 | kg/m3 | 308.15 | Densiti... |
| ρl | 724.82 | kg/m3 | 308.15 | Densiti... |
| ρl | 719.42 | kg/m3 | 313.15 | Densiti... |
| ρl | 718.08 | kg/m3 | 313.20 | Liquid-... |
| ρl | 713.96 | kg/m3 | 318.15 | Densiti... |
| ΔfusS | 46.18 | J/mol×K | 164.56 | NIST |
| csound,fluid | [103.70; 1086.92] | m/s | [288.15; 493.06] | |
|
T(K) Speed of sound in fluid (m/s) 200 400 600 800 1000 300 350 400 450 | ||||
| csound,fluid | 1086.92 | m/s | 288.15 | Mixing ... |
| csound,fluid | 1082.11 | m/s | 288.15 | Influen... |
| csound,fluid | 1086.62 | m/s | 288.15 | Mixing ... |
| csound,fluid | 1082.40 | m/s | 288.15 | Excess ... |
| csound,fluid | 1083.38 | m/s | 288.15 | Influen... |
| csound,fluid | 1083.38 | m/s | 288.15 | Influen... |
| csound,fluid | 1071.61 | m/s | 290.65 | Influen... |
| csound,fluid | 1070.62 | m/s | 290.65 | Influen... |
| csound,fluid | 1071.61 | m/s | 290.65 | Influen... |
| csound,fluid | 1060.05 | m/s | 293.15 | Influen... |
| csound,fluid | 1060.05 | m/s | 293.15 | Influen... |
| csound,fluid | 1063.43 | m/s | 293.15 | Mixing ... |
| csound,fluid | 1059.10 | m/s | 293.15 | Influen... |
| csound,fluid | 1059.40 | m/s | 293.15 | Excess ... |
| csound,fluid | 1047.83 | m/s | 295.65 | Influen... |
| csound,fluid | 1048.48 | m/s | 295.65 | Influen... |
| csound,fluid | 1048.48 | m/s | 295.65 | Influen... |
| csound,fluid | 1036.63 | m/s | 298.15 | Influen... |
| csound,fluid | 1036.10 | m/s | 298.15 | Excess ... |
| csound,fluid | 1036.94 | m/s | 298.15 | Influen... |
| csound,fluid | 1040.35 | m/s | 298.15 | Mixing ... |
| csound,fluid | 1036.94 | m/s | 298.15 | Influen... |
| csound,fluid | 1025.38 | m/s | 300.65 | Influen... |
| csound,fluid | 1025.42 | m/s | 300.65 | Influen... |
| csound,fluid | 1025.42 | m/s | 300.65 | Influen... |
| csound,fluid | 1024.33 | m/s | 301.65 | Influen... |
| csound,fluid | 1017.42 | m/s | 303.15 | Mixing ... |
| csound,fluid | 1014.04 | m/s | 303.15 | Influen... |
| csound,fluid | 1013.90 | m/s | 303.15 | Influen... |
| csound,fluid | 1013.00 | m/s | 303.15 | Excess ... |
| csound,fluid | 1012.81 | m/s | 303.15 | Influen... |
| csound,fluid | 1013.90 | m/s | 303.15 | Influen... |
| csound,fluid | 1024.70 | m/s | 303.19 | Speed o... |
| csound,fluid | 1001.35 | m/s | 305.65 | Influen... |
| csound,fluid | 1002.41 | m/s | 305.65 | Influen... |
| csound,fluid | 1002.70 | m/s | 305.65 | Influen... |
| csound,fluid | 1002.41 | m/s | 305.65 | Influen... |
| csound,fluid | 991.34 | m/s | 308.15 | Influen... |
| csound,fluid | 990.99 | m/s | 308.15 | Influen... |
| csound,fluid | 990.10 | m/s | 308.15 | Excess ... |
| csound,fluid | 989.90 | m/s | 308.15 | Influen... |
| csound,fluid | 990.99 | m/s | 308.15 | Influen... |
| csound,fluid | 994.68 | m/s | 308.15 | Mixing ... |
| csound,fluid | 988.60 | m/s | 308.16 | Speed o... |
| csound,fluid | 979.62 | m/s | 310.65 | Influen... |
| csound,fluid | 980.00 | m/s | 310.65 | Influen... |
| csound,fluid | 978.47 | m/s | 310.65 | Influen... |
| csound,fluid | 979.62 | m/s | 310.65 | Influen... |
| csound,fluid | 960.40 | m/s | 313.08 | Speed o... |
| csound,fluid | 967.03 | m/s | 313.15 | Influen... |
| csound,fluid | 968.16 | m/s | 313.15 | Influen... |
| csound,fluid | 968.63 | m/s | 313.15 | Influen... |
| csound,fluid | 968.16 | m/s | 313.15 | Influen... |
| csound,fluid | 956.80 | m/s | 315.65 | Influen... |
| csound,fluid | 956.80 | m/s | 315.65 | Influen... |
| csound,fluid | 955.73 | m/s | 315.65 | Influen... |
| csound,fluid | 957.31 | m/s | 315.65 | Influen... |
| csound,fluid | 946.03 | m/s | 318.15 | Influen... |
| csound,fluid | 944.34 | m/s | 318.15 | Influen... |
| csound,fluid | 945.54 | m/s | 318.15 | Influen... |
| csound,fluid | 945.54 | m/s | 318.15 | Influen... |
| csound,fluid | 942.70 | m/s | 318.19 | Speed o... |
| csound,fluid | 934.24 | m/s | 320.65 | Influen... |
| csound,fluid | 934.24 | m/s | 320.65 | Influen... |
| csound,fluid | 933.01 | m/s | 320.65 | Influen... |
| csound,fluid | 934.76 | m/s | 320.65 | Influen... |
| csound,fluid | 912.40 | m/s | 323.07 | Speed o... |
| csound,fluid | 922.97 | m/s | 323.15 | Influen... |
| csound,fluid | 922.97 | m/s | 323.15 | Influen... |
| csound,fluid | 923.46 | m/s | 323.15 | Influen... |
| csound,fluid | 921.93 | m/s | 323.15 | Influen... |
| csound,fluid | 904.70 | m/s | 328.21 | Speed o... |
| csound,fluid | 876.80 | m/s | 333.13 | Speed o... |
| csound,fluid | 855.10 | m/s | 338.07 | Speed o... |
| csound,fluid | 826.30 | m/s | 343.12 | Speed o... |
| csound,fluid | 797.50 | m/s | 348.24 | Speed o... |
| csound,fluid | 781.50 | m/s | 353.11 | Speed o... |
| csound,fluid | 748.60 | m/s | 358.22 | Speed o... |
| csound,fluid | 727.10 | m/s | 363.16 | Speed o... |
| csound,fluid | 707.10 | m/s | 368.17 | Speed o... |
| csound,fluid | 698.90 | m/s | 373.05 | Speed o... |
| csound,fluid | 668.30 | m/s | 378.05 | Speed o... |
| csound,fluid | 646.40 | m/s | 383.08 | Speed o... |
| csound,fluid | 627.50 | m/s | 388.11 | Speed o... |
| csound,fluid | 609.10 | m/s | 393.16 | Speed o... |
| csound,fluid | 580.10 | m/s | 398.11 | Speed o... |
| csound,fluid | 558.00 | m/s | 403.24 | Speed o... |
| csound,fluid | 537.60 | m/s | 408.23 | Speed o... |
| csound,fluid | 515.80 | m/s | 413.20 | Speed o... |
| csound,fluid | 494.40 | m/s | 418.24 | Speed o... |
| csound,fluid | 476.00 | m/s | 423.15 | Speed o... |
| csound,fluid | 453.80 | m/s | 428.24 | Speed o... |
| csound,fluid | 430.30 | m/s | 433.05 | Speed o... |
| csound,fluid | 405.80 | m/s | 438.19 | Speed o... |
| csound,fluid | 387.30 | m/s | 443.09 | Speed o... |
| csound,fluid | 360.30 | m/s | 448.23 | Speed o... |
| csound,fluid | 339.50 | m/s | 453.10 | Speed o... |
| csound,fluid | 313.60 | m/s | 458.19 | Speed o... |
| csound,fluid | 287.10 | m/s | 463.23 | Speed o... |
| csound,fluid | 265.00 | m/s | 468.18 | Speed o... |
| csound,fluid | 236.50 | m/s | 473.17 | Speed o... |
| csound,fluid | 206.10 | m/s | 478.08 | Speed o... |
| csound,fluid | 177.70 | m/s | 483.20 | Speed o... |
| csound,fluid | 142.80 | m/s | 488.11 | Speed o... |
| csound,fluid | 103.70 | m/s | 493.06 | Speed o... |
| γ | [0.01; 0.02] | N/m | [243.08; 393.13] | |
|
T(K) Surface Tension (N/m) 0.01 0.02 0.02 0.03 250 300 350 | ||||
| γ | 0.02 | N/m | 243.08 | Surface... |
| γ | 0.02 | N/m | 248.01 | Surface... |
| γ | 0.02 | N/m | 253.11 | Surface... |
| γ | 0.02 | N/m | 258.13 | Surface... |
| γ | 0.02 | N/m | 263.10 | Surface... |
| γ | 0.02 | N/m | 268.13 | Surface... |
| γ | 0.02 | N/m | 273.16 | Surface... |
| γ | 0.02 | N/m | 278.11 | Surface... |
| γ | 0.02 | N/m | 283.12 | Surface... |
| γ | 0.02 | N/m | 288.13 | Surface... |
| γ | 0.02 | N/m | 293.07 | Surface... |
| γ | 0.02 | N/m | 298.08 | Surface... |
| γ | 0.02 | N/m | 303.11 | Surface... |
| γ | 0.02 | N/m | 308.15 | Surface... |
| γ | 0.02 | N/m | 313.16 | Surface... |
| γ | 0.02 | N/m | 318.11 | Surface... |
| γ | 0.02 | N/m | 323.16 | Surface... |
| γ | 0.01 | N/m | 328.14 | Surface... |
| γ | 0.01 | N/m | 333.15 | Surface... |
| γ | 0.01 | N/m | 338.16 | Surface... |
| γ | 0.01 | N/m | 343.13 | Surface... |
| γ | 0.01 | N/m | 348.13 | Surface... |
| γ | 0.01 | N/m | 353.13 | Surface... |
| γ | 0.01 | N/m | 358.15 | Surface... |
| γ | 0.01 | N/m | 363.13 | Surface... |
| γ | 0.01 | N/m | 368.13 | Surface... |
| γ | 0.01 | N/m | 373.12 | Surface... |
| γ | 0.01 | N/m | 378.12 | Surface... |
| γ | 0.01 | N/m | 383.12 | Surface... |
| γ | 0.01 | N/m | 388.14 | Surface... |
| γ | 0.01 | N/m | 393.13 | Surface... |
| αdiff | [2.73e-08; 6.40e-08] | m2/s | [303.15; 493.15] | |
|
T(K) Thermal diffusivity (m2/s) 3.00e-8 4.00e-8 5.00e-8 6.00e-8 300 350 400 450 | ||||
| αdiff | 6.40e-08 | m2/s | 303.15 | Thermal... |
| αdiff | 6.17e-08 | m2/s | 313.15 | Thermal... |
| αdiff | 5.87e-08 | m2/s | 323.15 | Thermal... |
| αdiff | 5.70e-08 | m2/s | 333.15 | Thermal... |
| αdiff | 5.42e-08 | m2/s | 343.15 | Thermal... |
| αdiff | 5.26e-08 | m2/s | 353.15 | Thermal... |
| αdiff | 5.05e-08 | m2/s | 363.15 | Thermal... |
| αdiff | 4.83e-08 | m2/s | 373.15 | Thermal... |
| αdiff | 4.72e-08 | m2/s | 383.15 | Thermal... |
| αdiff | 4.64e-08 | m2/s | 393.15 | Thermal... |
| αdiff | 4.55e-08 | m2/s | 403.15 | Thermal... |
| αdiff | 4.48e-08 | m2/s | 413.15 | Thermal... |
| αdiff | 4.38e-08 | m2/s | 423.15 | Thermal... |
| αdiff | 4.31e-08 | m2/s | 433.15 | Thermal... |
| αdiff | 4.18e-08 | m2/s | 443.15 | Thermal... |
| αdiff | 4.05e-08 | m2/s | 453.15 | Thermal... |
| αdiff | 3.91e-08 | m2/s | 458.15 | Thermal... |
| αdiff | 3.86e-08 | m2/s | 463.15 | Thermal... |
| αdiff | 3.66e-08 | m2/s | 468.15 | Thermal... |
| αdiff | 3.55e-08 | m2/s | 473.15 | Thermal... |
| αdiff | 3.47e-08 | m2/s | 475.15 | Thermal... |
| αdiff | 3.36e-08 | m2/s | 477.15 | Thermal... |
| αdiff | 3.36e-08 | m2/s | 479.15 | Thermal... |
| αdiff | 3.30e-08 | m2/s | 481.15 | Thermal... |
| αdiff | 3.22e-08 | m2/s | 483.15 | Thermal... |
| αdiff | 3.07e-08 | m2/s | 485.15 | Thermal... |
| αdiff | 3.02e-08 | m2/s | 487.15 | Thermal... |
| αdiff | 2.95e-08 | m2/s | 489.15 | Thermal... |
| αdiff | 2.81e-08 | m2/s | 491.15 | Thermal... |
| αdiff | 2.73e-08 | m2/s | 493.15 | Thermal... |
Datasets
Mass density, kg/m3
| Fixed | Measured | |
|---|---|---|
| Temperature, K - Liquid | Pressure, kPa - Liquid | Mass density, kg/m3 - Liquid |
| 283.15 | 100.00 | 750.94 |
| 288.15 | 100.00 | 745.79 |
| 293.15 | 100.00 | 740.59 |
| 298.15 | 100.00 | 735.34 |
| 303.15 | 100.00 | 730.04 |
| 308.15 | 100.00 | 724.69 |
| 313.15 | 100.00 | 719.29 |
| 318.15 | 100.00 | 713.84 |
| 323.15 | 100.00 | 708.45 |
| 283.15 | 5000.00 | 756.29 |
| 288.15 | 5000.00 | 751.33 |
| 293.15 | 5000.00 | 746.34 |
| 298.15 | 5000.00 | 741.32 |
| 303.15 | 5000.00 | 736.27 |
| 308.15 | 5000.00 | 731.19 |
| 313.15 | 5000.00 | 726.07 |
| 318.15 | 5000.00 | 720.92 |
| 323.15 | 5000.00 | 715.73 |
| 283.15 | 10000.00 | 761.29 |
| 288.15 | 10000.00 | 756.52 |
| 293.15 | 10000.00 | 751.7 |
| 298.15 | 10000.00 | 746.89 |
| 303.15 | 10000.00 | 742.05 |
| 308.15 | 10000.00 | 737.18 |
| 313.15 | 10000.00 | 732.27 |
| 318.15 | 10000.00 | 727.36 |
| 323.15 | 10000.00 | 722.39 |
| 283.15 | 15000.00 | 765.93 |
| 288.15 | 15000.00 | 761.32 |
| 293.15 | 15000.00 | 756.68 |
| 298.15 | 15000.00 | 752.04 |
| 303.15 | 15000.00 | 747.37 |
| 308.15 | 15000.00 | 742.69 |
| 313.15 | 15000.00 | 737.97 |
| 318.15 | 15000.00 | 733.26 |
| 323.15 | 15000.00 | 728.51 |
| 283.15 | 20000.00 | 770.32 |
| 288.15 | 20000.00 | 765.86 |
| 293.15 | 20000.00 | 761.35 |
| 298.15 | 20000.00 | 756.85 |
| 303.15 | 20000.00 | 752.37 |
| 308.15 | 20000.00 | 747.84 |
| 313.15 | 20000.00 | 743.29 |
| 318.15 | 20000.00 | 738.74 |
| 323.15 | 20000.00 | 734.2 |
| 283.15 | 25000.00 | 774.48 |
| 288.15 | 25000.00 | 770.15 |
| 293.15 | 25000.00 | 765.77 |
| 298.15 | 25000.00 | 761.42 |
| 303.15 | 25000.00 | 757.05 |
| 308.15 | 25000.00 | 752.67 |
| 313.15 | 25000.00 | 748.27 |
| 318.15 | 25000.00 | 743.91 |
| 323.15 | 25000.00 | 739.51 |
| 283.15 | 30000.00 | 778.47 |
| 288.15 | 30000.00 | 774.24 |
| 293.15 | 30000.00 | 770.01 |
| 298.15 | 30000.00 | 765.75 |
| 303.15 | 30000.00 | 761.51 |
| 308.15 | 30000.00 | 757.26 |
| 313.15 | 30000.00 | 752.99 |
| 318.15 | 30000.00 | 748.75 |
| 323.15 | 30000.00 | 744.49 |
| 283.15 | 35000.00 | 782.29 |
| 288.15 | 35000.00 | 778.15 |
| 293.15 | 35000.00 | 774.0 |
| 298.15 | 35000.00 | 769.88 |
| 303.15 | 35000.00 | 765.74 |
| 308.15 | 35000.00 | 761.6 |
| 313.15 | 35000.00 | 757.46 |
| 318.15 | 35000.00 | 753.35 |
| 323.15 | 35000.00 | 749.2 |
| 283.15 | 40000.00 | 785.94 |
| 288.15 | 40000.00 | 781.89 |
| 293.15 | 40000.00 | 777.85 |
| 298.15 | 40000.00 | 773.81 |
| 303.15 | 40000.00 | 769.79 |
| 308.15 | 40000.00 | 765.75 |
| 313.15 | 40000.00 | 761.71 |
| 318.15 | 40000.00 | 757.7 |
| 323.15 | 40000.00 | 753.67 |
| 283.15 | 45000.00 | 789.45 |
| 288.15 | 45000.00 | 785.49 |
| 293.15 | 45000.00 | 781.54 |
| 298.15 | 45000.00 | 777.6 |
| 303.15 | 45000.00 | 773.65 |
| 308.15 | 45000.00 | 769.71 |
| 313.15 | 45000.00 | 765.77 |
| 318.15 | 45000.00 | 761.86 |
| 323.15 | 45000.00 | 757.92 |
| 283.15 | 50000.00 | 792.86 |
| 288.15 | 50000.00 | 788.97 |
| 293.15 | 50000.00 | 785.09 |
| 298.15 | 50000.00 | 781.23 |
| 303.15 | 50000.00 | 777.36 |
| 308.15 | 50000.00 | 773.49 |
| 313.15 | 50000.00 | 769.65 |
| 318.15 | 50000.00 | 765.83 |
| 323.15 | 50000.00 | 761.98 |
| 283.15 | 55000.00 | 796.13 |
| 288.15 | 55000.00 | 792.3 |
| 293.15 | 55000.00 | 788.51 |
| 298.15 | 55000.00 | 784.71 |
| 303.15 | 55000.00 | 780.95 |
| 308.15 | 55000.00 | 777.14 |
| 313.15 | 55000.00 | 773.39 |
| 318.15 | 55000.00 | 769.63 |
| 323.15 | 55000.00 | 765.85 |
| 283.15 | 60000.00 | 799.29 |
| 288.15 | 60000.00 | 795.55 |
| 293.15 | 60000.00 | 791.81 |
| 298.15 | 60000.00 | 788.1 |
| 303.15 | 60000.00 | 784.37 |
| 308.15 | 60000.00 | 780.65 |
| 313.15 | 60000.00 | 776.97 |
| 318.15 | 60000.00 | 773.29 |
| 323.15 | 60000.00 | 769.61 |
| 283.15 | 65000.00 | 802.37 |
| 288.15 | 65000.00 | 798.67 |
| 293.15 | 65000.00 | 795.0 |
| 298.15 | 65000.00 | 791.36 |
| 303.15 | 65000.00 | 787.7 |
| 308.15 | 65000.00 | 784.04 |
| 313.15 | 65000.00 | 780.41 |
| 318.15 | 65000.00 | 776.82 |
| 323.15 | 65000.00 | 773.22 |
| Reference | ||
Correlations
| Property | Value | Unit | Temperature (K) | Source |
|---|---|---|---|---|
| Pvap | [1.33; 202.64] | kPa | [243.08; 348.65] |
The Yaw...
|
| Equation | ln(Pvp) = A + B/(T + C) | |||
| Coefficient A | 1.53357e+01 | |||
| Coefficient B | -3.16987e+03 | |||
| Coefficient C | -3.24310e+01 | |||
| Temperature range, min. | 243.08 | |||
| Temperature range, max. | 348.65 | |||
|
T(K) Vapor pressure (kPa) 0 50 100 150 200 250 300 350 | ||||
| Pvap | 1.33 | kPa | 243.08 | Calculated Property |
| Pvap | 2.95 | kPa | 254.81 | Calculated Property |
| Pvap | 6.02 | kPa | 266.54 | Calculated Property |
| Pvap | 11.49 | kPa | 278.27 | Calculated Property |
| Pvap | 20.67 | kPa | 290.00 | Calculated Property |
| Pvap | 35.34 | kPa | 301.73 | Calculated Property |
| Pvap | 57.76 | kPa | 313.46 | Calculated Property |
| Pvap | 90.75 | kPa | 325.19 | Calculated Property |
| Pvap | 137.73 | kPa | 336.92 | Calculated Property |
| Pvap | 202.64 | kPa | 348.65 | Calculated Property |
| Pvap | [5.51e-04; 3384.37] | kPa | [164.55; 497.10] |
KDB Vap...
|
| Equation | ln(Pvp) = A + B/T + C*ln(T) + D*T^2 | |||
| Coefficient A | 6.49173e+01 | |||
| Coefficient B | -5.55710e+03 | |||
| Coefficient C | -7.60854e+00 | |||
| Coefficient D | 6.59025e-06 | |||
| Temperature range, min. | 164.55 | |||
| Temperature range, max. | 497.10 | |||
|
T(K) Vapor pressure (kPa) 0 500 1000 1500 2000 2500 3000 3500 200 300 400 500 | ||||
| Pvap | 5.51e-04 | kPa | 164.55 | Calculated Property |
| Pvap | 0.06 | kPa | 201.50 | Calculated Property |
| Pvap | 1.40 | kPa | 238.45 | Calculated Property |
| Pvap | 12.09 | kPa | 275.40 | Calculated Property |
| Pvap | 58.24 | kPa | 312.35 | Calculated Property |
| Pvap | 191.91 | kPa | 349.30 | Calculated Property |
| Pvap | 489.32 | kPa | 386.25 | Calculated Property |
| Pvap | 1044.38 | kPa | 423.20 | Calculated Property |
| Pvap | 1966.13 | kPa | 460.15 | Calculated Property |
| Pvap | 3384.37 | kPa | 497.10 | Calculated Property |
Similar Compounds
Find more compounds similar to Propane, 2-methoxy-2-methyl-.
Mixtures
- Benzene + Propane, 2-methoxy-2-methyl-
- Toluene + Propane, 2-methoxy-2-methyl-
- Propane, 2-methoxy-2-methyl- + Ethylbenzene
- Propane, 2-methoxy-2-methyl- + Pentane, 2,2,4-trimethyl-
- 2-Propanol, 2-methyl- + Propane, 2-methoxy-2-methyl-
- Propane, 2-methoxy-2-methyl- + Pentane, 3-methyl-
- n-Hexane + Propane, 2-methoxy-2-methyl-
- Propane, 2-methoxy-2-methyl- + Heptane
- Butane, 1-chloro- + Propane, 2-methoxy-2-methyl-
- Propane, 2-methoxy-2-methyl- + Butane, 2-chloro-
- Propane, 1-chloro-2-methyl- + Propane, 2-methoxy-2-methyl-
- 2-Pyrrolidinone, 1-methyl- + Propane, 2-methoxy-2-methyl-
- Propane, 2-methoxy-2-methyl- + 1-Hexyne
- Propane, 2-methoxy-2-methyl- + 2-Hexyne
- Propane, 2-methoxy-2-methyl- + o-Xylene
- Benzene, 1,3-dimethyl- + Propane, 2-methoxy-2-methyl-
- p-Xylene + Propane, 2-methoxy-2-methyl-
- Propane, 2-methoxy-2-methyl- + Ethane, 1,2-dichloro-
- Propane, 2-methoxy-2-methyl- + Cyclohexane
- Propane, 2-methoxy-2-methyl- + Acetone
Find more mixtures with Propane, 2-methoxy-2-methyl-.
Sources
- KDB Pure (Korean Thermophysical Properties Databank)
- KDB Vapor Pressure Data
- Crippen Method
- Influence of Temperature on Thermodynamic Properties of Methyl t-Butyl Ether (MTBE)+Gasoline Additives
- Kinematic Viscosities for Ether + Alkane Mixtures: Experimental Results and UNIFAC-VISCO Parameters
- Experimental and Predicted Viscosities of Binary Mixtures Containing Chlorinated and Oxygenated Compounds
- (Solid + liquid) phase equilibria of binary mixtures containing N-methyl-2-pyrrolidinone and ethers at atmospheric pressure
- Phase equilibria for binary systems of octane boosters with 2,2,4-trimethylpentane
- Determination of excess molar enthalpies of the ternary system methyl tert-butyl ether + 1-pentanol + nonane at 298.15K Analysis and comparison with predicted values of the UNIFAC model and some empirical methods
- Liquid-liquid equilibrium data of water with neohexane, methylcyclohexane, tert-butyl methyl ether, n-heptane and vapor -liquid-liquid equilibrium with methane
- Thermodynamics of binary mixtures containing N-methyl-2-pyrrolidinone VLE measurements for systems with ethers Comparison with the Mod. UNIFAC (Do) and DISQUAC models Predictions for VLE, GE m, HEm and SLE?
- Infinite dilution activity coefficient measurements by inert gas stripping method
- Thermodynamics of isomeric hexynes +MTBE binary mixtures
- (Liquid + liquid) equilibria for the quaternary system water + methyl tert-butyl ether + ethanol + benzene at 303.15K
- Liquid liquid equilibria for the MTBE+water + salts systems at 298.15K
- Influence of temperature on thermodynamics of ethers + xylenes
- Isothermal vapour liquid equilibria of binary systems of 1,2-dichloroethane with ethers
- Study of the effect of increasing the chain length of the alkane in excess molar enthalpies of mixtures containing methyl tert-butyl ether, 1-propanol, alkane
- Phase equilibria and interfacial tensions in the systems methyl tert-butyl ether + acetone + cyclohexane, methyl tert-butyl ether + acetone and methyl tert-butyl ether + cyclohexane
- Activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-butyl-1-methylpyrrolidinium trifluoromethanesulfonate using GLC
- Isothermal vapour liquid equilibria in the binary and ternary systems composed of tert-butyl methyl ether, 3,3-dimethyl-2-butanone and 2,2-dimethyl-1-propanol
- Activity coefficients at infinite dilution measurements for organic solutes and water in the 1-hexyloxymethyl-3-methyl-imidazolium and 1,3-dihexyloxymethyl-imidazolium bis(trifluoromethylsulfonyl)-imide ionic liquids The cation influence
- Study on the effect of increasing the chain length of the alkanol in excess molar enthalpies of mixtures containing 2-methoxy-2-methylpropane, 1-alkanol, decane
- Activity coefficients at infinite dilution of organic solutes in the ionic liquid 1-ethyl-3-methylimidazolium methanesulfonate
- Solubility of caprolactam in different organic solvents
- A novel static analytical apparatus for phase equilibrium measurements
- Liquid liquid equilibria at 298.15 K for ternary mixtures of methyl tert-butyl ether + methanol (or ethanol) + imidazolium-based ionic liquids at atmospheric pressure
- Atmospheric densities and interfacial tensions for 1- alkanol (1-butanol to 1-octanol) + water and ether (MTBE, ETBE, DIPE, TAME and THP) + water demixed mixtures.
- Phase equilibria of (water + propionic acid or butyric acid + 2-methoxy- 2-methylpropane) ternary systems at 298.2 K and 323.2 K
- Activity coefficients at infinite dilution of organic solvents and water in 1-butyl-3-methylimidazolium dicyanamide. A literature review of hexane/hex-1-ene separation
- Experimental and modeled volumetric behavior of linear and branched ethers
- Determination and modeling for solid-liquid phase equilibrium of ternary caprolactam + cyclohexanone oxime + methyl tert-butyl ether system
- A milliliter-scale setup for the efficient characterization of isothermal vapor-liquid equilibria using Raman spectroscopy
- High selective water/butan-1-ol separation on investigation of limiting activity coefficients with [P8,8,8,8][[NTf2] ionic liquid
- Separation of binary mixtures based on limiting activity coefficients data using specific ammonium-based ionic liquid and modelling of thermodynamic functions
- Thermodynamics and activity coefficients at infinite dilution for organic solutes in the ionic liquid 1-butyl-1-methylpyrrolidinium dicyanamide
- New ionic liquid [P4,4,4,4][NTf2] in bio-butanol extraction on investigation of limiting activity coefficients
- Liquid-liquid equilibrium measurement and thermodynamics modeling for the systems water + thioglycolic acid + isopropyl ether/methyl tert-butyl ether at 298.15 and 308.15 K
- Ammonium ionic liquids in extraction of bio-butan-1-ol from water phase using activity coefficients at infinite dilution
- Liquid-liquid equilibrium data and thermophysical properties for ternary systems composed of water, acetic acid and different solvents
- Excess molar enthalpies of the ternary mixtures: methyl tert-butyl ether +2-methylpentane + (n-decane or n-dodecane) at the temperature 298.15 K
- Excess molar enthalpies of the ternary mixtures: (methyl tert-butyl ether or 2-methyltetrahydrofuran + cyclohexane + 2,2,4- trimethylpentane} at the temperature 298.15 K
- Densities and derived thermodynamic properties of the binary systems of 1,1-dimethylethyl methyl ether with allyl methacrylate, butyl methacrylate, methacrylic acid, and vinyl acetate at T = (298.15 and 308.15) K
- Excess molar enthalpies of the ternary mixtures: {tetrahydrofuran or 2-methyltetrahydrofuran + methyl tert-butyl ether + n-dodecane} at the temperature 298.15 K
- Quaternary (liquid + liquid) equilibria for (water + 1,1-dimethylethyl methyl ether + diisopropyl ether + toluene) at the temperature 298.15 K
- Quaternary (liquid + liquid) equilibria for (water + 2-propanol + 1,1-dimethylethyl methyl ether + diisopropyl ether) at the temperature 298.15 K
- Excess molar enthalpies of the ternary mixtures: (tetrahydrofuran or 2-methyltetrahydrofuran + methyl tert-butyl ether + n-octane) at the temperature 298.15 K
- (Liquid + liquid) phase equilibria of 1-alkyl-3-methylimidazolium methylsulfate with alcohols, or ethers, or ketones
- Excess molar enthalpies of the ternary mixtures (1-hexene + tetrahydrofuran or 2-methyltetrahydrofuran + methyl tert-butyl ether) at the temperature 298.15K.
- Excess enthalpy, density, and speed of sound determination for the ternary mixture (methyl tert-butyl ether + 1-butanol + n-hexane)
- (Vapor + liquid) equilibrium of the binary mixtures formed by acetonitrile with selected compounds at 95.5 kPa
- Thermodynamics of biofuels: Excess enthalpies for binary mixtures involving ethyl 1,1-dimethylethyl ether and hydrocarbons at different temperatures using a new flow calorimeter
- Activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid triethylsulphonium bis(trifluoromethylsulfonyl)imide
- Measurements of activity coefficients at infinite dilution of aliphatic and aromatic hydrocarbons, alcohols, thiophene, tetrahydrofuran, MTBE, and water in ionic liquid [BMIM][SCN] using GLC
- Volumetric behaviour of binary liquid systems composed of toluene, isooctane, and methyl tert-butyl ether at temperatures from (298.15 to 328.15) K
- Activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 4-methyl-N-butyl-pyridinium bis(trifluoromethylsulfonyl)-imide
- Gas liquid chromatography measurements of activity coefficients at infinite dilution of various organic solutes and water in tri-iso-butylmethylphosphonium tosylate ionic liquid
- Volumetric behaviour of the ternary liquid system composed of methyl tert-butyl ether, toluene, and isooctane at temperatures from (298.15 to 328.15) K: Experimental data and correlation
- Measurements of activity coefficients at infinite dilution of organic solutes and water in 1-propyl-1-methylpiperidinium bis{(trifluoromethyl)sulfonyl}imide ionic liquid using g.l.c.
- Measurements of activity coefficients at infinite dilution of organic compounds and water in isoquinolinium-based ionic liquid [C8iQuin][NTf2] using GLC
- Activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-ethyl-3-methylimidazolium tetracyanoborate
- Volumetric and viscometric properties of binary mixtures of {methyl tert-butyl ether (MTBE) + alcohol} at several temperatures and p = 0.1 MPa: Experimental results and application of the ERAS model
- Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid 1-(3-hydroxypropyl)pyridinium bis(trifluoromethylsulfonyl)-amide
- Thermodynamics and activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-butyl-1-methylpyrrolidinium tetracyanoborate
- Interactions of volatile organic compounds with the ionic liquid 1-ethyl-3-methylimidazolium tetracyanoborate
- Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid 4-(2-methoxyethyl)-4-methylmorpholinium bis(trifluoromethylsulfonyl)-amide
- Measurements of activity coefficients at infinite dilution for organic solutes and water in the ionic liquid 1-hexyl-3-methylimidazolium tetracyanoborate
- Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid 1-(2-methoxyethyl)-1-methylpiperidinium bis(trifluoromethylsulfonyl)-amide
- Measurements of activity coefficients at infinite dilution for organic solutes and water in the ionic liquid 1-ethyl-3-methylimidazolium methanesulfonate
- Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid 1-(2-methoxyethyl)-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)-amide
- Isothermal (vapor + liquid) equilibria and excess enthalpy data of {1-hexene + methyl butyl ether (MBE)} and {1-hexene + methyl tert-butyl ether (MTBE)} binary systems at several temperatures
- Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid 4-(2-methoxyethyl)-4-methylmorpholinium trifluorotris(perfluoroethyl)phosphate
- Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid 1-(2-methoxyethyl)- 1-methylpiperidinium trifluorotris(perfluoroethyl)phosphate
- Interactions of volatile organic compounds with the ionic liquids 1-butyl-1-methylpyrrolidinium tetracyanoborate and 1-butyl-1-methylpyrrolidinium bis(oxalato)borate
- Physicochemical properties and activity coefficients at infinite dilution for organic solutes and water in a novel bicyclic guanidinium superbase-derived protic ionic liquid
- Experimental and theoretical study on infinite dilution activity coefficients of various solutes in piperidinium ionic liquids
- Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid 1-(2-methoxyethyl)- 1-methylpyrrolidinium trifluorotris(perfluoroethyl)phosphate
- Measurements of activity coefficients at infinite dilution for organic solutes and water in N-hexylisoquinolinium thiocyanate, [HiQuin][SCN] using GLC
- Measurement of critical temperatures and critical pressures for binary mixtures of methyl tert-butyl ether (MTBE) + alcohol and MTBE + alkane
- Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid 1-(2-hydroxyethyl)- 3-methylimidazolium trifluorotris(perfluoroethyl)phosphate
- Infinite dilution activity coefficients of volatile organic compounds in two ionic liquids composed of the tris(pentafluoroethyl) trifluorophosphate ([FAP]) anion and a functionalized cation
- Experimental and theoretical excess molar enthalpies of ternary and binary mixtures containing 2-Methoxy-2-Methylpropane, 1-propanol, heptane
- Measurements of activity coefficients at infinite dilution for organic solutes and water in the ionic liquid 1-butyl-1-methylpyrrolidinium tricyanomethanide
- Thermodynamics and activity coefficients at infinite dilution for organic solutes and water in the ionic liquid 1-butyl-1-methylmorpholinium tricyanomethanide
- The study of activity coefficients at infinite dilution for organic solutes and water in 1-butyl-4-methylpyridinium dicyanamide, [B4MPy][DCA] using GLC
- Contribution to study of the thermodynamics properties of mixtures containing 2-methoxy-2-methylpropane, alkanol, alkane
- Measurement and correlation of critical properties for binary mixtures and ternary mixtures containing gasoline additives
- Thermodynamics and activity coefficients at infinite dilution for organic solutes, water and diols in the ionic liquid choline bis(trifluoromethylsulfonyl)imide
- Solubility and solution thermodynamics of sorbic acid in eight pure organic solvents
- Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid 4-(3-hydroxypropyl)-4-methylmorpholinium bis(trifluoromethylsulfonyl)-amide
- Activity coefficients at infinite dilution for organic solutes and water in 1-ethyl-1-methylpyrrolidinium lactate
- Activity coefficients at infinite dilution, physicochemical and thermodynamic properties for organic solutes and water in the ionic liquid ethyl-dimethyl-(2-methoxyethyl)ammonium trifluorotris-(perfluoroethyl)phosphate
- Volumetric behavior of the ternary system (methyl tert-butyl ether + methylbenzene + butan-1-ol) and its binary sub-system (methyl tert-butyl ether + butan-1-ol) within the temperature range (298.15 to 328.15) K
- A 1-alkylcyanopyridinium-based ionic liquid in the separation processes
- Thermodynamics and selectivity of separation based on activity coefficients at infinite dilution of various solutes in 1-allyl-3-methylimidazolium bis{(trifluoromethyl)sulfonyl}imide ionic liquid
- Separation of aliphatic from aromatic hydrocarbons and sulphur compounds from fuel based on measurements of activity coefficients at infinite dilution for organic solutes and water in the ionic liquid N,N-diethyl-N-methyl-N-(2-methoxy-ethyl)ammonium bis(trifluoromethylsulfonyl)imide
- Thermodynamics and limiting activity coefficients measurements for organic solutes and water in the ionic liquid 1-dodecyl-3-methylimidzolium bis(trifluoromethylsulfonyl) imide
- Solubility measurement and correlation of the form A of ibrutinib in organic solvents from 278.15 to 323.15 K
- Separation of binary mixtures hexane/hex-1-ene, cyclohexane/cyclohexene and ethylbenzene/styrene based on limiting activity coefficients
- Separation of water/butan-1-ol based on activity coefficients at infinite dilution in 1,3-didecyl-2-methylimidazolium dicyanamide ionic liquid
- Separation of binary mixtures hexane/hex-1-ene, cyclohexane/cyclohexene and ethylbenzene/styrene based on gamma infinity data measurements
- Activity coefficients at infinite dilution and physicochemical properties for organic solutes and water in the ionic liquid trihexyl-tetradecyl-phosphonium tricyanomethanide
- Thermodynamic study of molecular interaction-selectivity in separation processes based on limiting activity coefficients
- Liquid-liquid equilibrium for ternary systems of water + 2,2,3,3-tetrafluoro-1-propanol + isopropyl ether/tert-butyl methyl ether at 298.2, 308.2 K
- The use of ionic liquids for separation of binary hydrocarbons mixtures based on gamma infinity data measurements
- Liquid-liquid equilibria for azeotropic mixture of methyl tert-butyl ether and methanol with ionic liquids at different temperatures
- Liquid-liquid equilibrium study for ternary systems of water + propargyl alcohol + solvents at 308.2 K: Measurement and thermodynamic modelling
- Excess molar enthalpies of the ternary mixtures: methyl tert-butyl ether + 3-methylpentane + (n-decane or n-dodecane) at 298.15K
- Isothermal vapor-liquid equilibrium of binary and ternary systems of anisole, hexane, and toluene and ternary system of methyl tert-butyl ether, hexane, and toluene
- sothermal and Isobaric Vapor-Liquid Equilibrium and Excess Molar Enthalpy of the Binary Mixtures of 2-Methoxy-2-methylpropane + 2-Methyl-2-butanol or + 2-Butanol
- Solubility of Amorphous Clopidogrel Hydrogen Sulfate in Different Pure Solvents
- Solubility of Agomelatine Crystal Form I and Form II in Pure Solvents and (Isopropanol + Water) Mixtures
- Activity Coefficients at Infinite Dilution of Organic Solutes and Water in Tributylethylphosphonium Diethylphosphate Using Gas Liquid Chromatography: Thermodynamic Properties of Mixtures Containing Ionic Liquids
- Quaternary, Ternary, and Binary LLE Measurements for 2-Methoxy-2-methylpropane + Furfural + Acetic Acid + Water at Temperatures between 298 and 307 K
- Speed of Sound Measurements of 2-Methoxy-2-methylpropane in the Temperature Range of 293.15 and 673.15 K and for Pressures up to 10 MPa
- Liquid-Liquid Equilibria of Ionic Liquids-Water-Acetic Acid Mixtures
- Measurements and Thermodynamic Models for the Separation of 2-Methoxyethanol and Water Azeotropic Mixtures via Different Solvents
- Solid-Liquid Phase Equilibrium and Thermodynamic Properties of Olaparib in Selected Organic Solvents and (Tetrahydrofuran + MTBE, Acetonitrile + Isopropyl Alcohol) Binary Solvent Mixtures
- Solubility Measurement and Thermodynamic Modeling of N-(4-Methylphenyl-Z-3-chloro-2-(phenylthio)propenamide in 12 Pure Solvents at Temperatures Ranging from 278.15 to 318.15 K
- Physicochemical Properties of LiFSI Solutions II: LiFSI with Water, MTBE, and Anisole
- Vapor-Liquid Equilibrium Measurements of Ether Alcohol Blends for Investigation on Reformulated Gas
- Solubility Measurement and Correlation of Probenecid in 12 Pure Organic Solvents and Thermodynamic Properties of Mixing of Solutions
- Measurement and Correlation of the Solubility of Dienogest in Twelve Pure and Water + Methanol Binary Solvents at Temperatures from 273.15 to 318.15 K
- Solubility of Imidazoles in Ethers
- Mixing Properties for the Ternary Mixture Methyl tert-Butyl Ether + 1-Butanol + Decane at 298.15 K
- Vapor-Liquid Equilibria for the Binary Systems of Dimethoxymethane with Some Fuel Oxygenates
- Oxygenated Gasoline Additives: Saturated Heat Capacities between (277 and 355) K
- Excess Molar Volumes and Surface Tensions of Xylene with Isopropyl Ether or Methyl tert-Butyl Ether at 298.15 K
- Solubility of a-Carotene in Binary Solvents Formed by Some Hydrocarbons with tert-Butyl Methyl Ether and with tert-Amyl Methyl Ether
- Liquid-Liquid Equilibrium of MTBE + Ethanol + Water and MTBE + 1-Hexanol + Water over the Temperature Range of 288.15 to 308.15 K
- Experimental and Predicted Excess Molar Enthalpies of the Ternary System tert-Butyl Methyl Ether + 1-Pentanol + Decane at 298.15 K
- Vapor-Liquid Equilibrium Measurements of MTBE and TAME with Toluene
- Measurement of Activity Coefficients at Infinite Dilution of Benzene, Toluene, Ethanol, Esters, Ketones, and Ethers at Various Temperatures in Water Using the Dilutor Technique
- Vapor-Liquid Equilibria for the Ternary Systems of Methyl tert-Butyl Ether + Methanol + Methylcyclohexane and Methyl tert-Butyl Ether + Methanol + n-Heptane and Constituent Binary Systems at 313.15 K
- Liquid-Liquid Equilibria for the Ternary Systems Water + 3-Methyl-2-cyclopentenone with Ethyl Acetate or Methyl tert-Butyl Ether at 293.2 K
- Densities and Viscosities for the Ternary Systems of Methyl tert-Butyl Ether + Methanol + Benzene and Methyl tert-Butyl Ether + Methanol + Toluene and Their Sub-binary Systems at 298.15 K
- Surface Tension of Dimethoxymethane and Methyl tert-Butyl Ether
- Phase Equilibrium Properties of Binary and Ternary Mixtures Containing 1,1-Dimethylethyl Methyl Ether, 1-Propanol, and Hexane at T ) 313.15 K
- Liquid-Liquid Equilibria of the Ternary System Water + Acetic Acid + Methyl tert-Butyl Ether
- Activity Coefficients at Infinite Dilution Measurements for Organic Solutes and Water in the Ionic Liquid 1-Butyl-3-methyl-pyridinium Trifluoromethanesulfonate
- Solubility of Artemisinin in Different Single and Binary Solvent Mixtures Between (284.15 and 323.15) K and NRTL Interaction Parameters
- Determination of Activity Coefficients at Infinite Dilution of 35 Solutes in the Ionic Liquid, 1-Butyl-3-methylimidazolium Tosylate, Using Gas-Liquid Chromatography
- Density, Viscosity, Vapor-Liquid Equilibrium, and Excess Molar Enthalpy of [Chloroform + Methyl tert-Butyl Ether]
- Measurements of Activity Coefficients at Infinite Dilution for Organic Solutes and Water in the Ionic Liquid 1-Butyl-1-methylpiperydinium Thiocyanate
- Interactions of Volatile Organic Compounds with the Ionic Liquid 1-Butyl-1-methylpyrrolidinium Dicyanamide
- Excess Molar Enthalpies of Ternary and Binary Mixtures Containing 2-Methoxy-2-Methylpropane, Ethanol, and Nonane
- Excess Volumes of Ternary Mixtures 2,2,4-Trimethylpentane + Diisopropyl Ether or Methyl tert-Butyl Ether + Methanol, Ethanol, or 1-Propanol at 298.15 K
- Determination of Henry's Law Constants Using Internal Standards with Benchmark Values
- Liquid Liquid Equilibria for the Ternary System 2-Methoxy-2-methylpropane + Phenol + Water
- Measurements of Liquid Liquid Equilibria for the Quaternary System 2-Methoxy-2-methylpropane + Phenol + Hydroquinone + Water at 313.15 K
- Thermal Diffusivity of 2-Methoxy-2-methylpropane at Temperatures from (303.15 to 493.15) K and Pressures from (1.5 to 10) MPa
- Experimental Determination and Correlation of Liquid Liquid Equilibria for the Ternary System 2-Methoxy-2-methylpropane + o-Cresol + Water at 298.15 K and 313.15 K
- Densities and Viscosities of MTBE + Heptane or Octane at p ) 0.1 MPa from (273.15 to 363.15) K
- Densities and Viscosities of MTBE + Nonane or Decane at p = 0.1 MPa from (273.15 to 363.15) K
- Phase Equilibria of (1-Ethyl-3-methylimidazolium Ethylsulfate + Hydrocarbon, + Ketone, and + Ether) Binary Systems
- Excess Enthalpy, Density, and Speed of Sound for the Ternary Mixture Methyl tert-Butyl Ether (1) + Butan-1-ol (2) + Octane (3)
- Measurement and Correlation of Liquid-Liquid Equilibrium Data for Water + Acetic Acid + Methyl tert-Butyl Ether + NaCl
- Excess Molar Enthalpies of Ternary and Binary Mixtures Containing 2-Methoxy-2-methylpropane, 1-Propanol, and Nonane
- Isobaric Vapor-Liquid Equilibria at 101.32 kPa and Densities, Speeds of Sound, and Refractive Indices at 298.15 K for MTBE or DIPE or TAME + 1-Propanol Binary Systems
- Vapor-Liquid Equilibria and Interfacial Tensions of the System Ethanol + 2-Methoxy-2-methylpropane
- Mixing Properties for Binary Liquid Mixtures of Methyl tert-Butyl Ether with Propylamine and Dipropylamine at Temperatures from (288.15 to 308.15) K
- Activity Coefficients at Infinite Dilution Measurements for Organic Solutes and Water in the Ionic Liquid 1-Hexyl-3-methylimidazolium Thiocyanate
- Joback Method
- KDB
- Aqueous Solubility Prediction Method
- Estimated Solubility Method
- McGowan Method
- NIST Webbook
- The Yaws Handbook of Vapor Pressure
Note: Cheméo is only indexing the data, follow the source links to retrieve the latest data. The source is also providing more information like the publication year, authors and more.
Take the time to validate and double check the source of the data.
Outlier This icon means
that the value is more than 2 standard deviations away from the
property mean.