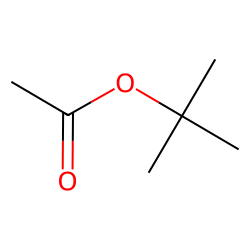
Physical Properties
Property
Value
Unit
Source
Δc H°liquid -3521.50 ± 1.30
kJ/mol
NIST
Δf G° -231.44
kJ/mol
Joback Calculated Property
Δf H°gas -516.50 ± 1.30
kJ/mol
NIST
Δf H°liquid -554.50 ± 1.30
kJ/mol
NIST
Δfus H° 6.67
kJ/mol
Joback Calculated Property
Δvap H° [38.00; 38.03]
kJ/mol
Δvap H° 38.03 ± 0.21
kJ/mol
NIST
Δvap H° 38.00
kJ/mol
NIST
Δvap H° 38.00 ± 0.20
kJ/mol
NIST
Δvap H° 38.00 ± 0.20
kJ/mol
NIST
log 10 WS-1.31
Crippen Calculated Property
log Poct/wat 1.348
Crippen Calculated Property
McVol 102.840
ml/mol
McGowan Calculated Property
Pc 3040.00
kPa
Critica...
Inp [636.00; 701.40]
Inp 636.00
NIST
Inp 641.00
NIST
Inp 686.00
NIST
Inp 701.00
NIST
Inp 639.00
NIST
Inp 636.00
NIST
Inp 640.00
NIST
Inp 701.40
NIST
Inp 687.00
NIST
Inp 687.00
NIST
Inp 676.00
NIST
Inp 686.00
NIST
Inp 676.00
NIST
Inp 687.00
NIST
Inp 687.00
NIST
Inp 636.00
NIST
I [845.00; 912.50]
I 888.00
NIST
I Outlier
NIST
I 912.50
NIST
I 878.00
NIST
I 893.00
NIST
I 893.00
NIST
I 912.50
NIST
I 893.00
NIST
Tboil [369.00; 371.05]
K
Tboil 371.05
K
Isobari...
Tboil 369.15
K
Isobari...
Tboil 370.70
K
NIST
Tboil 369.00
K
NIST
Tboil 369.00 ± 3.00
K
NIST
Tboil 369.20 ± 2.00
K
NIST
Tboil 371.00 ± 0.40
K
NIST
Tc 599.15
K
Joback Calculated Property
Tfus 231.96
K
Joback Calculated Property
Vc 0.385
m3 /kmol
Joback Calculated Property
Temperature Dependent Properties
Property
Value
Unit
Temperature (K)
Source
Cp,gas [201.96; 261.33]
J/mol×K
[409.74; 599.15]
Cp,gas 201.96
J/mol×K
409.74
Joback Calculated Property
Cp,gas 213.08
J/mol×K
441.31
Joback Calculated Property
Cp,gas 223.69
J/mol×K
472.88
Joback Calculated Property
Cp,gas 233.81
J/mol×K
504.44
Joback Calculated Property
Cp,gas 243.45
J/mol×K
536.01
Joback Calculated Property
Cp,gas 252.62
J/mol×K
567.58
Joback Calculated Property
Cp,gas 261.33
J/mol×K
599.15
Joback Calculated Property
Cp,liquid 231.00
J/mol×K
298.15
NIST
η [0.0002947; 0.0047319]
Pa×s
[231.96; 409.74]
η 0.0047319
Pa×s
231.96
Joback Calculated Property
η 0.0022923
Pa×s
261.59
Joback Calculated Property
η 0.0012870
Pa×s
291.22
Joback Calculated Property
η 0.0008038
Pa×s
320.85
Joback Calculated Property
η 0.0005436
Pa×s
350.48
Joback Calculated Property
η 0.0003908
Pa×s
380.11
Joback Calculated Property
η 0.0002947
Pa×s
409.74
Joback Calculated Property
Δvap H 36.70
kJ/mol
340.00
NIST
Pvap [101.30; 101.33]
kPa
[369.15; 371.05]
Pvap 101.30
kPa
369.15
Isobari...
Pvap 101.33
kPa
371.05
Isobari...
n 0 1.38400
298.15
Isobari...
csound,fluid [1040.00; 1091.00]
m/s
[298.15; 313.15]
csound,fluid 1091.00
m/s
298.15
Densiti...
csound,fluid 1078.00
m/s
303.15
Densiti...
csound,fluid 1049.00
m/s
308.15
Densiti...
csound,fluid 1040.00
m/s
313.15
Densiti...
Correlations
Similar Compounds
Find more compounds similar to Acetic acid, 1,1-dimethylethyl ester .
Mixtures
2-Propanol, 2-methyl- + Acetic acid, 1,1-dimethylethyl ester
Acetic acid, 1,1-dimethylethyl ester + Simvastatin
Benzyl alcohol + Acetic acid, 1,1-dimethylethyl ester
2-Propanol, 1,3-dichloro- + Acetic acid, 1,1-dimethylethyl ester
Acetic acid, 1,1-dimethylethyl ester + Benzene, chloro-
2-Propanol, 2-methyl- + Acetic acid, 1,1-dimethylethyl ester + Benzene, chloro-
Acetic acid, 1,1-dimethylethyl ester + Ethane, 1,2-dichloro-
Acetic acid, 1,1-dimethylethyl ester + Methyl Alcohol
Benzene + Acetic acid, 1,1-dimethylethyl ester
Toluene + Acetic acid, 1,1-dimethylethyl ester
Acetic acid, 1,1-dimethylethyl ester + Ethylbenzene
Acetic acid, 1,1-dimethylethyl ester + Lovastatin
Acetic acid, 1,1-dimethylethyl ester + Acetic acid + Water
Acetic acid, 1,1-dimethylethyl ester + Water
Sources
KDB Vapor Pressure Data Crippen Method Crippen Method Isobaric vapor-liquid equilibria for the binary systems isobutyl alcohol + isobutyl acetate and tert-butyl alcohol + tert-butyl acetate at 20 and 101.3 kPa Solubility and limiting activity coefficient of simvastatin in different organic solvents Densities, viscosities, speed of sound, and IR spectroscopic studies of binary mixtures of tert-butyl acetate with benzene, methylbenzene, and ethylbenzene at T = (298.15 and 308.15) K The study of excess molar volumes and related properties for binary mixtures containing benzyl alcohol and 1,3-dichloro-2-propanol with vinyl acetate, ethyl acetate and t-butyl acetate at T = 293.15 to 313.15 K and P = 0.087 MPa Isobaric Vapor-Liquid Equilibrium for Binary and Ternary Systems of tert-Butanol + tert-Butyl Acetate + Chlorobenzene at 101.33 kPa Isobaric Vapor-Liquid Equilibrium for the Binary Systems of 1,2-Dichloroethane + sec-Butyl Acetate, n-Propyl Acetate, and tert-Butyl Acetate at 101.3 kPa Critical Point and Vapor Pressure Measurements for 17 Compounds by a Low Residence Time Flow Method Liquid-Liquid Equilibria for the Acetic Acid + Water + Amyl Acetate and Acetic Acid + Water + 2-Methyl Ethyl Acetate Ternary Systems Solubility of Lovastatin in Ethyl Acetate, Propyl Acetate, Isopropyl Acetate, Butyl Acetate, sec-Butyl Acetate, Isobutyl Acetate, tert-Butyl Acetate, and 2-Butanone, between (285 and 313) K Densities, Sound Speed, and IR Studies of (Methanol + 1-Acetoxybutane) and (Methanol + 1,1-Dimethylethyl Ester) at (298.15, 303.15, 308.15, and 313.15) K Joback Method KDB McGowan Method NIST Webbook The Yaws Handbook of Vapor Pressure
Note: Cheméo is only indexing the data, follow the source links to retrieve the latest data. The source is also providing more information like the publication year, authors and more.
Take the time to validate and double check the source of the data.
Outlier