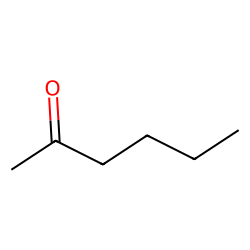
Physical Properties
| Property | Value | Unit | Source |
|---|---|---|---|
| ω | 0.3920 | KDB | |
| ΔcH°liquid | -3754.02 ± 0.96 | kJ/mol | NIST |
| ΔfG° | -129.28 | kJ/mol | Joback Calculated Property |
| ΔfH°gas | -279.80 ± 1.10 | kJ/mol | NIST |
| ΔfH°liquid | -322.00 ± 1.00 | kJ/mol | NIST |
| ΔfusH° | 12.89 | kJ/mol | Joback Calculated Property |
| ΔvapH° | [42.20; 43.15] | kJ/mol |
|
| ΔvapH° | 43.15 | kJ/mol | NIST |
| ΔvapH° | 43.10 ± 0.10 | kJ/mol | NIST |
| ΔvapH° | 43.03 | kJ/mol | NIST |
| ΔvapH° | 43.10 ± 0.10 | kJ/mol | NIST |
| ΔvapH° | 43.00 ± 0.30 | kJ/mol | NIST |
| ΔvapH° | 42.90 | kJ/mol | NIST |
| ΔvapH° | 42.22 ± 0.08 | kJ/mol | NIST |
| ΔvapH° | 42.20 ± 0.10 | kJ/mol | NIST |
| IE | [9.20; 9.44] | eV |
|
| IE | 9.35 ± 0.06 | eV | NIST |
| IE | 9.24 ± 0.02 | eV | NIST |
| IE | 9.33 ± 0.01 | eV | NIST |
| IE | 9.20 | eV | NIST |
| IE | 9.36 ± 0.02 | eV | NIST |
| IE | 9.37 ± 0.02 | eV | NIST |
| IE | 9.34 ± 0.03 | eV | NIST |
| IE | 9.44 ± 0.03 | eV | NIST |
| IE | 9.38 | eV | NIST |
| log10WS | [-0.80; -0.80] |
|
|
| log10WS | -0.80 | Aq. Sol... | |
| log10WS | -0.80 | Estimat... | |
| logPoct/wat | 1.766 | Crippen Calculated Property | |
| McVol | 96.970 | ml/mol | McGowan Calculated Property |
| NFPA Fire | 3 | KDB | |
| NFPA Health | 2 | KDB | |
| Pc | [3320.00; 3320.00] | kPa |
|
| Pc | 3320.00 | kPa | KDB |
| Pc | 3320.00 ± 80.00 | kPa | NIST |
| ρc | 267.42 ± 3.00 | kg/m3 | NIST |
| Inp | [726.00; 807.00] |
|
|
| Inp | 767.90 | NIST | |
| Inp | 767.07 | NIST | |
| Inp | 766.66 | NIST | |
| Inp | 765.86 | NIST | |
| Inp | 766.82 | NIST | |
| Inp | 767.68 | NIST | |
| Inp | 768.37 | NIST | |
| Inp | 768.70 | NIST | |
| Inp | 769.32 | NIST | |
| Inp | 769.68 | NIST | |
| Inp | 771.05 | NIST | |
| Inp | 772.24 | NIST | |
| Inp | 774.21 | NIST | |
| Inp | 788.05 | NIST | |
| Inp | 787.01 | NIST | |
| Inp | 787.84 | NIST | |
| Inp | 789.11 | NIST | |
| Inp | 789.29 | NIST | |
| Inp | 789.92 | NIST | |
| Inp | 790.50 | NIST | |
| Inp | 791.67 | NIST | |
| Inp | 792.39 | NIST | |
| Inp | 793.70 | NIST | |
| Inp | 795.42 | NIST | |
| Inp | 797.53 | NIST | |
| Inp | 769.69 | NIST | |
| Inp | 770.63 | NIST | |
| Inp | 770.60 | NIST | |
| Inp | 767.41 | NIST | |
| Inp | 765.83 | NIST | |
| Inp | 790.62 | NIST | |
| Inp | 791.12 | NIST | |
| Inp | 791.21 | NIST | |
| Inp | 790.92 | NIST | |
| Inp | 792.67 | NIST | |
| Inp | 769.00 | NIST | |
| Inp | 766.90 | NIST | |
| Inp | 766.80 | NIST | |
| Inp | 766.97 | NIST | |
| Inp | 767.10 | NIST | |
| Inp | 767.30 | NIST | |
| Inp | 768.04 | NIST | |
| Inp | 767.70 | NIST | |
| Inp | 769.00 | NIST | |
| Inp | 767.00 | NIST | |
| Inp | 767.00 | NIST | |
| Inp | 768.00 | NIST | |
| Inp | 775.00 | NIST | |
| Inp | 777.00 | NIST | |
| Inp | 774.00 | NIST | |
| Inp | 774.00 | NIST | |
| Inp | 766.60 | NIST | |
| Inp | 772.00 | NIST | |
| Inp | Outlier 731.40 | NIST | |
| Inp | 791.30 | NIST | |
| Inp | 792.30 | NIST | |
| Inp | 794.30 | NIST | |
| Inp | 770.00 | NIST | |
| Inp | Outlier 742.00 | NIST | |
| Inp | 773.00 | NIST | |
| Inp | 751.00 | NIST | |
| Inp | 759.00 | NIST | |
| Inp | 747.00 | NIST | |
| Inp | 763.00 | NIST | |
| Inp | 764.00 | NIST | |
| Inp | 764.00 | NIST | |
| Inp | Outlier 738.00 | NIST | |
| Inp | Outlier 728.00 | NIST | |
| Inp | Outlier 736.00 | NIST | |
| Inp | 778.00 | NIST | |
| Inp | 745.60 | NIST | |
| Inp | Outlier 726.00 | NIST | |
| Inp | Outlier 727.00 | NIST | |
| Inp | 745.00 | NIST | |
| Inp | 752.00 | NIST | |
| Inp | 747.00 | NIST | |
| Inp | 752.00 | NIST | |
| Inp | 772.00 | NIST | |
| Inp | 761.00 | NIST | |
| Inp | 770.00 | NIST | |
| Inp | 770.00 | NIST | |
| Inp | 771.00 | NIST | |
| Inp | 788.00 | NIST | |
| Inp | 768.00 | NIST | |
| Inp | 775.00 | NIST | |
| Inp | 790.00 | NIST | |
| Inp | 788.00 | NIST | |
| Inp | 802.00 | NIST | |
| Inp | 789.00 | NIST | |
| Inp | 760.60 | NIST | |
| Inp | 787.50 | NIST | |
| Inp | 802.00 | NIST | |
| Inp | 801.00 | NIST | |
| Inp | 771.00 | NIST | |
| Inp | 792.00 | NIST | |
| Inp | 792.00 | NIST | |
| Inp | 793.00 | NIST | |
| Inp | 798.00 | NIST | |
| Inp | 792.00 | NIST | |
| Inp | 793.00 | NIST | |
| Inp | 770.00 | NIST | |
| Inp | 760.00 | NIST | |
| Inp | 792.00 | NIST | |
| Inp | 793.00 | NIST | |
| Inp | 789.00 | NIST | |
| Inp | 763.00 | NIST | |
| Inp | 792.00 | NIST | |
| Inp | 747.00 | NIST | |
| Inp | 753.00 | NIST | |
| Inp | 755.00 | NIST | |
| Inp | 792.00 | NIST | |
| Inp | 803.00 | NIST | |
| Inp | 750.00 | NIST | |
| Inp | 789.00 | NIST | |
| Inp | 807.00 | NIST | |
| Inp | 769.00 | NIST | |
| Inp | 768.00 | NIST | |
| Inp | 769.00 | NIST | |
| Inp | 769.00 | NIST | |
| Inp | 771.00 | NIST | |
| Inp | 771.00 | NIST | |
| Inp | 768.00 | NIST | |
| Inp | 768.00 | NIST | |
| Inp | 767.00 | NIST | |
| Inp | 758.00 | NIST | |
| Inp | 786.00 | NIST | |
| Inp | 789.00 | NIST | |
| Inp | 791.00 | NIST | |
| Inp | 768.00 | NIST | |
| Inp | 789.00 | NIST | |
| Inp | 792.00 | NIST | |
| Inp | 792.00 | NIST | |
| Inp | 790.00 | NIST | |
| Inp | 787.00 | NIST | |
| Inp | 788.00 | NIST | |
| Inp | 790.00 | NIST | |
| Inp | 786.00 | NIST | |
| Inp | 786.00 | NIST | |
| Inp | 789.00 | NIST | |
| Inp | 789.00 | NIST | |
| Inp | 761.00 | NIST | |
| Inp | 800.00 | NIST | |
| Inp | 770.00 | NIST | |
| Inp | 761.00 | NIST | |
| Inp | 765.00 | NIST | |
| Inp | 792.00 | NIST | |
| Inp | 793.00 | NIST | |
| Inp | 759.00 | NIST | |
| Inp | 761.00 | NIST | |
| Inp | 761.00 | NIST | |
| Inp | 761.00 | NIST | |
| Inp | 790.00 | NIST | |
| Inp | 792.00 | NIST | |
| Inp | 793.00 | NIST | |
| Inp | 747.00 | NIST | |
| Inp | 772.00 | NIST | |
| Inp | 775.00 | NIST | |
| Inp | 767.00 | NIST | |
| Inp | 760.00 | NIST | |
| Inp | 779.00 | NIST | |
| Inp | 797.00 | NIST | |
| Inp | 791.00 | NIST | |
| Inp | 778.00 | NIST | |
| Inp | 747.00 | NIST | |
| Inp | 768.00 | NIST | |
| Inp | 795.00 | NIST | |
| Inp | 791.00 | NIST | |
| Inp | 788.00 | NIST | |
| Inp | 769.00 | NIST | |
| Inp | 756.00 | NIST | |
| Inp | 782.00 | NIST | |
| Inp | 791.00 | NIST | |
| Inp | 791.00 | NIST | |
| Inp | 767.00 | NIST | |
| Inp | 772.00 | NIST | |
| Inp | 786.00 | NIST | |
| Inp | 790.00 | NIST | |
| Inp | 767.00 | NIST | |
| Inp | 788.00 | NIST | |
| Inp | 787.00 | NIST | |
| Inp | 806.00 | NIST | |
| Inp | 768.00 | NIST | |
| Inp | 771.00 | NIST | |
| Inp | 768.00 | NIST | |
| Inp | 768.00 | NIST | |
| Inp | 768.00 | NIST | |
| Inp | 771.00 | NIST | |
| Inp | 787.00 | NIST | |
| Inp | 769.00 | NIST | |
| Inp | 769.00 | NIST | |
| Inp | 787.00 | NIST | |
| Inp | 786.00 | NIST | |
| Inp | 792.00 | NIST | |
| Inp | 790.00 | NIST | |
| Inp | 761.00 | NIST | |
| Inp | 772.00 | NIST | |
| Inp | 788.00 | NIST | |
| Inp | 769.00 | NIST | |
| Inp | 771.00 | NIST | |
| I | [1060.00; 1124.00] |
|
|
| I | 1098.02 | NIST | |
| I | Outlier 1116.32 | NIST | |
| I | 1113.00 | NIST | |
| I | 1097.20 | NIST | |
| I | 1102.20 | NIST | |
| I | 1107.60 | NIST | |
| I | 1082.00 | NIST | |
| I | 1079.00 | NIST | |
| I | 1074.00 | NIST | |
| I | 1077.00 | NIST | |
| I | Outlier 1060.00 | NIST | |
| I | Outlier 1124.00 | NIST | |
| I | 1083.00 | NIST | |
| I | 1083.00 | NIST | |
| I | 1083.00 | NIST | |
| I | 1094.00 | NIST | |
| I | 1083.00 | NIST | |
| I | 1084.00 | NIST | |
| I | 1082.00 | NIST | |
| I | 1089.00 | NIST | |
| I | 1081.00 | NIST | |
| I | 1089.00 | NIST | |
| I | 1082.00 | NIST | |
| I | 1092.00 | NIST | |
| I | 1095.00 | NIST | |
| I | 1078.00 | NIST | |
| I | 1083.00 | NIST | |
| I | 1102.00 | NIST | |
| I | 1112.00 | NIST | |
| I | 1100.00 | NIST | |
| I | 1069.00 | NIST | |
| I | 1075.00 | NIST | |
| I | 1083.00 | NIST | |
| I | 1074.00 | NIST | |
| I | 1077.00 | NIST | |
| I | 1100.00 | NIST | |
| I | 1077.00 | NIST | |
| I | 1088.00 | NIST | |
| I | 1107.00 | NIST | |
| I | 1068.00 | NIST | |
| I | 1078.00 | NIST | |
| I | 1070.00 | NIST | |
| I | 1095.00 | NIST | |
| I | 1100.00 | NIST | |
| I | 1076.00 | NIST | |
| I | 1080.00 | NIST | |
| I | 1087.00 | NIST | |
| I | 1097.00 | NIST | |
| I | 1070.00 | NIST | |
| I | 1090.00 | NIST | |
| I | 1078.00 | NIST | |
| I | 1070.00 | NIST | |
| I | 1098.02 | NIST | |
| I | 1107.60 | NIST | |
| S°liquid | 308.10 | J/mol×K | NIST |
| Tboil | [397.15; 422.90] | K |
|
| Tboil | 400.70 | K | KDB |
| Tboil | 400.69 | K | Isobari... |
| Tboil | 401.20 | K | NIST |
| Tboil | 400.70 | K | NIST |
| Tboil | 400.30 ± 0.50 | K | NIST |
| Tboil | 397.15 ± 3.00 | K | NIST |
| Tboil | 400.15 ± 3.00 | K | NIST |
| Tboil | 401.15 ± 1.00 | K | NIST |
| Tboil | 400.95 ± 0.50 | K | NIST |
| Tboil | 399.00 ± 4.00 | K | NIST |
| Tboil | 400.75 ± 0.50 | K | NIST |
| Tboil | 400.35 ± 0.50 | K | NIST |
| Tboil | 400.95 ± 1.00 | K | NIST |
| Tboil | 399.65 ± 1.50 | K | NIST |
| Tboil | 399.15 ± 2.00 | K | NIST |
| Tboil | 402.15 ± 2.00 | K | NIST |
| Tboil | 401.15 ± 5.00 | K | NIST |
| Tboil | 397.65 ± 5.00 | K | NIST |
| Tboil | 400.15 ± 2.00 | K | NIST |
| Tboil | 400.70 ± 0.50 | K | NIST |
| Tboil | 401.15 ± 5.00 | K | NIST |
| Tboil | 400.30 ± 0.30 | K | NIST |
| Tboil | 400.15 ± 2.00 | K | NIST |
| Tboil | 398.65 ± 2.00 | K | NIST |
| Tboil | 398.65 ± 3.00 | K | NIST |
| Tboil | 399.55 ± 2.00 | K | NIST |
| Tboil | 400.15 ± 2.00 | K | NIST |
| Tboil | 399.65 ± 2.00 | K | NIST |
| Tboil | 400.45 ± 2.00 | K | NIST |
| Tboil | 401.65 ± 1.00 | K | NIST |
| Tboil | 400.35 ± 0.50 | K | NIST |
| Tboil | 400.35 ± 0.30 | K | NIST |
| Tboil | 399.40 ± 1.00 | K | NIST |
| Tboil | 400.15 ± 1.00 | K | NIST |
| Tboil | Outlier 422.90 ± 10.00 | K | NIST |
| Tboil | 400.15 ± 1.00 | K | NIST |
| Tboil | 400.52 ± 0.50 | K | NIST |
| Tboil | 400.15 ± 1.00 | K | NIST |
| Tc | [586.60; 587.00] | K |
|
| Tc | 587.00 | K | KDB |
| Tc | 586.60 ± 0.40 | K | NIST |
| Tc | 587.00 | K | NIST |
| Tc | 587.00 ± 0.50 | K | NIST |
| Tfus | [216.25; 217.60] | K |
|
| Tfus | 217.03 | K | Aq. Sol... |
| Tfus | 217.60 | K | KDB |
| Tfus | 216.25 ± 0.30 | K | NIST |
| Ttriple | [217.69; 218.42] | K |
|
| Ttriple | 218.42 | K | Thermod... |
| Ttriple | 217.69 ± 0.05 | K | NIST |
| Vc | 0.378 | m3/kmol | Joback Calculated Property |
Temperature Dependent Properties
| Property | Value | Unit | Temperature (K) | Source |
|---|---|---|---|---|
| Cp,gas | [177.72; 232.63] | J/mol×K | [390.55; 568.08] | |
|
T(K) Ideal gas heat capacity (J/mol×K) 180 190 200 210 220 230 400 450 500 550 | ||||
| Cp,gas | 177.72 | J/mol×K | 390.55 | Joback Calculated Property |
| Cp,gas | 187.80 | J/mol×K | 420.14 | Joback Calculated Property |
| Cp,gas | 197.51 | J/mol×K | 449.73 | Joback Calculated Property |
| Cp,gas | 206.83 | J/mol×K | 479.31 | Joback Calculated Property |
| Cp,gas | 215.79 | J/mol×K | 508.90 | Joback Calculated Property |
| Cp,gas | 224.39 | J/mol×K | 538.49 | Joback Calculated Property |
| Cp,gas | 232.63 | J/mol×K | 568.08 | Joback Calculated Property |
| Cp,liquid | [213.30; 213.40] | J/mol×K | [298.15; 298.15] | |
| Cp,liquid | 213.40 | J/mol×K | 298.15 | NIST |
| Cp,liquid | 213.30 | J/mol×K | 298.15 | NIST |
| η | [0.0003010; 0.0038104] | Pa×s | [207.31; 390.55] | |
|
T(K) Dynamic viscosity (Pa×s) 0 5.00e-4 1.00e-3 1.50e-3 2.00e-3 2.50e-3 3.00e-3 3.50e-3 4.00e-3 250 300 350 | ||||
| η | 0.0038104 | Pa×s | 207.31 | Joback Calculated Property |
| η | 0.0019023 | Pa×s | 237.85 | Joback Calculated Property |
| η | 0.0011124 | Pa×s | 268.39 | Joback Calculated Property |
| η | 0.0007258 | Pa×s | 298.93 | Joback Calculated Property |
| η | 0.0005126 | Pa×s | 329.47 | Joback Calculated Property |
| η | 0.0003840 | Pa×s | 360.01 | Joback Calculated Property |
| η | 0.0003010 | Pa×s | 390.55 | Joback Calculated Property |
| ΔfusH | [14.90; 14.90] | kJ/mol | [217.69; 217.70] | |
| ΔfusH | 14.90 | kJ/mol | 217.69 | NIST |
| ΔfusH | 14.90 | kJ/mol | 217.70 | NIST |
| ΔfusH | 14.90 | kJ/mol | 217.70 | NIST |
| ΔvapH | [36.10; 53.80] | kJ/mol | [308.00; 550.00] | |
|
T(K) Enthalpy of vaporization at a given temperature (kJ/mol) 35 40 45 50 55 400 500 | ||||
| ΔvapH | 42.50 ± 0.10 | kJ/mol | 308.00 | NIST |
| ΔvapH | 41.60 ± 0.10 | kJ/mol | 323.00 | NIST |
| ΔvapH | 40.70 ± 0.10 | kJ/mol | 338.00 | NIST |
| ΔvapH | 53.80 | kJ/mol | 340.00 | NIST |
| ΔvapH | 40.10 ± 0.10 | kJ/mol | 348.00 | NIST |
| ΔvapH | 43.80 | kJ/mol | 351.00 | NIST |
| ΔvapH | 40.80 | kJ/mol | 352.00 | NIST |
| ΔvapH | 39.50 ± 0.10 | kJ/mol | 358.00 | NIST |
| ΔvapH | 41.50 | kJ/mol | 368.50 | NIST |
| ΔvapH | 39.00 | kJ/mol | 380.00 | NIST |
| ΔvapH | 36.35 | kJ/mol | 400.70 | NIST |
| ΔvapH | 43.10 | kJ/mol | 400.70 | NIST |
| ΔvapH | 36.70 | kJ/mol | 472.00 | NIST |
| ΔvapH | 36.10 | kJ/mol | 550.00 | NIST |
| n0 | [1.40080; 1.40080] | [293.13; 293.15] | ||
| n0 | 1.40080 | 293.13 | Isobari... | |
| n0 | 1.40080 | 293.15 | Isobari... | |
| ρl | [788.45; 816.00] | kg/m3 | [288.00; 318.15] | |
|
T(K) Liquid Density (kg/m3) 790 795 800 805 810 815 290 300 310 | ||||
| ρl | 816.00 | kg/m3 | 288.00 | KDB |
| ρl | 815.72 | kg/m3 | 288.15 | Thermal... |
| ρl | 811.00 | kg/m3 | 293.20 | Ternary... |
| ρl | 806.70 | kg/m3 | 298.15 | Thermal... |
| ρl | 806.37 | kg/m3 | 298.20 | Liquid-... |
| ρl | 797.60 | kg/m3 | 308.15 | Thermal... |
| ρl | 788.45 | kg/m3 | 318.15 | Thermal... |
| ΔfusS | [68.42; 68.44] | J/mol×K | [217.69; 217.70] | |
| ΔfusS | 68.44 | J/mol×K | 217.69 | NIST |
| ΔfusS | 68.42 | J/mol×K | 217.70 | NIST |
Correlations
| Property | Value | Unit | Temperature (K) | Source |
|---|---|---|---|---|
| Pvap | [1.33; 202.66] | kPa | [302.50; 424.77] |
The Yaw...
|
| Equation | ln(Pvp) = A + B/(T + C) | |||
| Coefficient A | 1.38679e+01 | |||
| Coefficient B | -2.82795e+03 | |||
| Coefficient C | -9.42620e+01 | |||
| Temperature range, min. | 302.50 | |||
| Temperature range, max. | 424.77 | |||
|
T(K) Vapor pressure (kPa) 0 50 100 150 200 300 350 400 | ||||
| Pvap | 1.33 | kPa | 302.50 | Calculated Property |
| Pvap | 3.06 | kPa | 316.09 | Calculated Property |
| Pvap | 6.39 | kPa | 329.67 | Calculated Property |
| Pvap | 12.31 | kPa | 343.26 | Calculated Property |
| Pvap | 22.16 | kPa | 356.84 | Calculated Property |
| Pvap | 37.63 | kPa | 370.43 | Calculated Property |
| Pvap | 60.82 | kPa | 384.01 | Calculated Property |
| Pvap | 94.17 | kPa | 397.60 | Calculated Property |
| Pvap | 140.44 | kPa | 411.18 | Calculated Property |
| Pvap | 202.66 | kPa | 424.77 | Calculated Property |
| Pvap | [1.50e-03; 2927.28] | kPa | [217.35; 587.05] |
KDB Vap...
|
| Equation | ln(Pvp) = A + B/T + C*ln(T) + D*T^2 | |||
| Coefficient A | 4.75253e+00 | |||
| Coefficient B | -5.04800e+03 | |||
| Coefficient C | 2.29627e+00 | |||
| Coefficient D | -8.15597e-06 | |||
| Temperature range, min. | 217.35 | |||
| Temperature range, max. | 587.05 | |||
|
T(K) Vapor pressure (kPa) 0 500 1000 1500 2000 2500 3000 300 400 500 | ||||
| Pvap | 1.50e-03 | kPa | 217.35 | Calculated Property |
| Pvap | 0.08 | kPa | 258.43 | Calculated Property |
| Pvap | 1.30 | kPa | 299.51 | Calculated Property |
| Pvap | 10.74 | kPa | 340.58 | Calculated Property |
| Pvap | 53.97 | kPa | 381.66 | Calculated Property |
| Pvap | 188.45 | kPa | 422.74 | Calculated Property |
| Pvap | 498.85 | kPa | 463.82 | Calculated Property |
| Pvap | 1062.21 | kPa | 504.89 | Calculated Property |
| Pvap | 1896.72 | kPa | 545.97 | Calculated Property |
| Pvap | 2927.28 | kPa | 587.05 | Calculated Property |
Similar Compounds
Find more compounds similar to 2-Hexanone.
Mixtures
- 2-Hexanone + 2-Pyrrolidinone, 1-methyl-
- Phenol, 2-methyl- + 2-Hexanone + Water
- 2-Hexanone + Phenol, 3-methyl- + Water
- p-Cresol + 2-Hexanone + Water
- 2-Hexanone + Water
- 2-Hexanone + Phenol + Water
- 2-Hexanone + Hydroquinone + Water
- Cyclohexanol + 2-Hexanone
- Cyclohexanol + 2-Hexanone + Nonane
- 2-Hexanone + 1-Pentanol
- 2-Hexanone + 1-Pentanol + Nonane
- 2-Hexanone + Anisole
- 2-Hexanone + Anisole + 1-Pentanol
- 2-Hexanone + Sodium sulfate + Water
- 2-Hexanone + sodium chloride + Water
Sources
- KDB Vapor Pressure Data
- Crippen Method
- A relative headspace method for Henry s constants of volatile organic compounds
- Liquid-liquid equilibria in the ternary systems water + cresols + methyl butyl ketone at 298.2 and 313.2 K: experimental data and correlation
- A thermodynamic study of the ketoreductase-catalyzed reduction of 2-alkanones in non-aqueous solvents
- Thermodynamics of binary mixtures of N-methyl-2-pyrrolidinone and ketone. Experimental results and modelling of the solid-liquid equilibrium and vapou-liquid equilibrium. The Modified UNIFAC (Do) model characterization
- (Liquid + liquid) phase equilibria of 1-alkyl-3-methylimidazolium methylsulfate with alcohols, or ethers, or ketones
- Measurements of activity coefficients at infinite dilution of organic solutes and water in 1-propyl-1-methylpiperidinium bis{(trifluoromethyl)sulfonyl}imide ionic liquid using g.l.c.
- Activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-ethyl-3-methylimidazolium tetracyanoborate
- Physicochemical properties and activity coefficients at infinite dilution for organic solutes and water in a novel bicyclic guanidinium superbase-derived protic ionic liquid
- Experimental and theoretical study on infinite dilution activity coefficients of various solutes in piperidinium ionic liquids
- Measurements of activity coefficients at infinite dilution for organic solutes and water in N-hexylisoquinolinium thiocyanate, [HiQuin][SCN] using GLC
- The study of activity coefficients at infinite dilution for organic solutes and water in 1-butyl-4-methylpyridinium dicyanamide, [B4MPy][DCA] using GLC
- Activity coefficients at infinite dilution for organic solutes and water in 1-ethyl-1-methylpyrrolidinium lactate
- Separation of aliphatic from aromatic hydrocarbons and sulphur compounds from fuel based on measurements of activity coefficients at infinite dilution for organic solutes and water in the ionic liquid N,N-diethyl-N-methyl-N-(2-methoxy-ethyl)ammonium bis(trifluoromethylsulfonyl)imide
- Solubility measurement, model evaluation and thermodynamic analysis of rivaroxaban polymorphs in organic solvents
- Liquid-liquid equilibrium for methyl butyl ketone + o-, m-, p-cresol + water ternary systems and COSMO-SAC predictions
- Ternary and Quaternary Liquid-Liquid Equilibria for Systems of Methyl Butyl Ketone + Water + Hydroquinone + Phenol at 313.2 K and Atmospheric Pressure
- Activity Coefficients at Infinite Dilution of Organic Solutes and Water in Tributylethylphosphonium Diethylphosphate Using Gas Liquid Chromatography: Thermodynamic Properties of Mixtures Containing Ionic Liquids
- Thermal and Volumetric Properties of Some C5 and C6 Alkanones at Infinite Dilution in Water
- Air-Water Partitioning of C5 and C6 Alkanones: Measurement, Critical Compilation, Correlation, and Recommended Data
- Isobaric Vapor-Liquid Equilibria of the Ternary System Methylbutyl Ketone + Nonane + Cyclohexanol
- Determination of Henry s Law Constants and Activity Coefficients at Infinite Dilution of Flavor Compounds in Water at 298 K with a Gas-Chromatographic Method
- Isobaric Vapor-Liquid Equilibria of the Ternary System Methylbutyl Ketone + 1-Pentanol + Nonane
- Henry s Constants of 1-Alkanols and 2-Ketones in Salt Solutions
- Determination of Henry's Law Constants Using Internal Standards with Benchmark Values
- Liquid Liquid Equilibria for Ternary Systems: Methyl Butyl Ketone + Phenol + Water and Methyl Butyl Ketone + Hydroquinone + Water at 298.15 K and 323.15 K
- Phase Equilibria of (1-Ethyl-3-methylimidazolium Ethylsulfate + Hydrocarbon, + Ketone, and + Ether) Binary Systems
- Isobaric Vapor-Liquid Equilibria of the Ternary System Methylbutyl Ketone + 1-Pentanol + Anisole
- Joback Method
- KDB
- Aqueous Solubility Prediction Method
- Estimated Solubility Method
- McGowan Method
- NIST Webbook
- The Yaws Handbook of Vapor Pressure
Note: Cheméo is only indexing the data, follow the source links to retrieve the latest data. The source is also providing more information like the publication year, authors and more.
Take the time to validate and double check the source of the data.
Outlier This icon means
that the value is more than 2 standard deviations away from the
property mean.