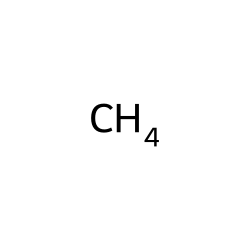
Contents
- Physical Properties
- Temperature Dependent Properties
- Datasets
- Correlations
- Similar Compounds
- Mixtures
- Sources
Physical Properties
| Property | Value | Unit | Source |
|---|---|---|---|
| ω | 0.0110 | KDB | |
| PAff | 543.50 | kJ/mol | NIST |
| Tig | 813.15 | K | KDB |
| BasG | 520.60 | kJ/mol | NIST |
| ΔcH°gas | [-891.80; -890.16] | kJ/mol |
|
| ΔcH°gas | -890.70 ± 0.40 | kJ/mol | NIST |
| ΔcH°gas | -890.35 ± 0.30 | kJ/mol | NIST |
| ΔcH°gas | -891.80 ± 1.10 | kJ/mol | NIST |
| ΔcH°gas | -890.16 ± 0.30 | kJ/mol | NIST |
| μ | 0.00 | debye | KDB |
| LFL | 5.00 | % in Air | KDB |
| UFL | 15.00 | % in Air | KDB |
| ΔfG° | -50.87 | kJ/mol | KDB |
| Rg | 1.1230 | KDB | |
| ΔfH°gas | [-74.90; -73.40] | kJ/mol |
|
| ΔfH°gas | -74.90 | kJ/mol | KDB |
| ΔfH°gas | -74.60 ± 0.30 | kJ/mol | NIST |
| ΔfH°gas | -74.50 ± 0.40 | kJ/mol | NIST |
| ΔfH°gas | -74.85 ± 0.31 | kJ/mol | NIST |
| ΔfH°gas | -73.40 ± 1.10 | kJ/mol | NIST |
| IE | [12.51; 13.60] | eV |
|
| IE | 12.61 ± 0.01 | eV | NIST |
| IE | 12.61 ± 0.01 | eV | NIST |
| IE | 12.60 ± 0.40 | eV | NIST |
| IE | 12.63 ± 0.02 | eV | NIST |
| IE | Outlier 13.60 | eV | NIST |
| IE | 12.75 ± 0.02 | eV | NIST |
| IE | 12.82 ± 0.02 | eV | NIST |
| IE | 12.60 | eV | NIST |
| IE | 12.60 | eV | NIST |
| IE | 12.80 | eV | NIST |
| IE | 12.64 | eV | NIST |
| IE | 12.94 ± 0.04 | eV | NIST |
| IE | 12.51 | eV | NIST |
| IE | 12.51 | eV | NIST |
| IE | 12.62 ± 0.01 | eV | NIST |
| IE | 12.78 | eV | NIST |
| IE | 12.75 | eV | NIST |
| IE | 12.70 | eV | NIST |
| IE | 12.99 ± 0.05 | eV | NIST |
| IE | 12.75 ± 0.05 | eV | NIST |
| IE | 12.90 | eV | NIST |
| IE | 12.70 | eV | NIST |
| IE | 12.55 ± 0.05 | eV | NIST |
| IE | 12.70 ± 0.01 | eV | NIST |
| IE | 12.71 ± 0.02 | eV | NIST |
| IE | 13.00 ± 0.02 | eV | NIST |
| IE | Outlier 13.60 | eV | NIST |
| IE | Outlier 13.60 ± 0.10 | eV | NIST |
| IE | Outlier 13.60 | eV | NIST |
| log10WS | [-1.11; -0.90] |
|
|
| log10WS | -1.11 | Aq. Sol... | |
| log10WS | -0.90 | Estimat... | |
| logPoct/wat | 0.636 | Crippen Calculated Property | |
| McVol | 24.950 | ml/mol | McGowan Calculated Property |
| NFPA Fire | 4 | KDB | |
| NFPA Health | 1 | KDB | |
| Pc | 4599.00 | kPa | KDB |
| Ptriple | 11.70 | kPa | KDB |
| S°gas | 188.66 ± 0.42 | J/mol×K | NIST |
| Tboil | [109.20; 112.00] | K |
|
| Tboil | 111.67 | K | KDB |
| Tboil | 111.70 | K | NIST |
| Tboil | 111.65 ± 0.00 | K | NIST |
| Tboil | 111.60 ± 0.20 | K | NIST |
| Tboil | 111.70 | K | NIST |
| Tboil | 111.70 ± 0.30 | K | NIST |
| Tboil | 111.65 | K | NIST |
| Tboil | 111.65 ± 0.07 | K | NIST |
| Tboil | 112.00 ± 0.50 | K | NIST |
| Tboil | 112.00 ± 0.50 | K | NIST |
| Tboil | 112.00 ± 0.50 | K | NIST |
| Tboil | 111.50 ± 0.50 | K | NIST |
| Tboil | 110.20 ± 1.00 | K | NIST |
| Tboil | Outlier 109.20 ± 1.00 | K | NIST |
| Tc | [173.70; 199.70] | K |
|
| Tc | 190.56 | K | KDB |
| Tc | 190.56 ± 0.01 | K | NIST |
| Tc | 190.55 ± 0.01 | K | NIST |
| Tc | 190.58 | K | NIST |
| Tc | 190.53 ± 0.05 | K | NIST |
| Tc | 190.55 ± 0.01 | K | NIST |
| Tc | 190.60 | K | NIST |
| Tc | 190.78 ± 0.40 | K | NIST |
| Tc | 190.55 ± 0.05 | K | NIST |
| Tc | 190.55 | K | NIST |
| Tc | 190.53 ± 0.00 | K | NIST |
| Tc | 190.78 ± 0.40 | K | NIST |
| Tc | 190.57 ± 0.10 | K | NIST |
| Tc | 190.60 ± 0.15 | K | NIST |
| Tc | 190.30 ± 0.40 | K | NIST |
| Tc | 190.59 ± 0.40 | K | NIST |
| Tc | 190.70 ± 0.40 | K | NIST |
| Tc | 191.07 ± 0.60 | K | NIST |
| Tc | 190.30 ± 0.50 | K | NIST |
| Tc | 190.30 ± 0.60 | K | NIST |
| Tc | 191.40 ± 1.00 | K | NIST |
| Tc | Outlier 173.70 ± 20.00 | K | NIST |
| Tc | Outlier 199.70 ± 10.00 | K | NIST |
| Tc | 197.50 ± 7.00 | K | NIST |
| Tfus | [85.70; 91.20] | K |
|
| Tfus | 90.69 | K | KDB |
| Tfus | 90.83 | K | Aq. Sol... |
| Tfus | Outlier 85.70 ± 0.20 | K | NIST |
| Tfus | 90.60 ± 0.30 | K | NIST |
| Tfus | 91.20 ± 2.00 | K | NIST |
| Tfus | 90.60 ± 0.20 | K | NIST |
| Tfus | 90.50 ± 0.20 | K | NIST |
| Ttriple | 90.69 | K | KDB |
| Vc | [0.099; 0.100] | m3/kmol |
|
| Vc | 0.099 | m3/kmol | KDB |
| Vc | 0.099 | m3/kmol | NIST |
| Vc | 0.099 | m3/kmol | NIST |
| Vc | 0.100 ± 0.001 | m3/kmol | NIST |
| Zc | 0.2861960 | KDB | |
| Zra | 0.29 | KDB |
Temperature Dependent Properties
| Property | Value | Unit | Temperature (K) | Source |
|---|---|---|---|---|
| Cp,gas | 34.92 ± 0.25 | J/mol×K | 279.00 | NIST |
| ΔfusH | 0.94 | kJ/mol | 90.70 | NIST |
| ΔsubH | [9.20; 10.00] | kJ/mol | [63.00; 84.00] | |
|
T(K) Enthalpy of sublimation at a given temperature (kJ/mol) 9.2 9.4 9.6 9.8 10 65 70 75 80 | ||||
| ΔsubH | 9.70 | kJ/mol | 63.00 | NIST |
| ΔsubH | 9.70 | kJ/mol | 72.00 | NIST |
| ΔsubH | 9.20 | kJ/mol | 72.00 | NIST |
| ΔsubH | 9.62 | kJ/mol | 77.50 | NIST |
| ΔsubH | 10.00 | kJ/mol | 84.00 | NIST |
| ΔvapH | [4.00; 8.70] | kJ/mol | [99.00; 180.00] | |
|
T(K) Enthalpy of vaporization at a given temperature (kJ/mol) 4 5 6 7 8 9 100 150 | ||||
| ΔvapH | 8.50 ± 0.10 | kJ/mol | 99.00 | NIST |
| ΔvapH | 8.52 | kJ/mol | 99.54 | NIST |
| ΔvapH | 8.60 | kJ/mol | 101.00 | NIST |
| ΔvapH | 8.60 | kJ/mol | 105.00 | NIST |
| ΔvapH | 7.65 | kJ/mol | 108.15 | Enthalp... |
| ΔvapH | 8.60 | kJ/mol | 109.00 | NIST |
| ΔvapH | 8.18 | kJ/mol | 111.60 | KDB |
| ΔvapH | 8.17 | kJ/mol | 111.70 | NIST |
| ΔvapH | 8.20 | kJ/mol | 112.00 | NIST |
| ΔvapH | 8.05 | kJ/mol | 118.04 | Enthalp... |
| ΔvapH | 7.91 | kJ/mol | 123.15 | Enthalp... |
| ΔvapH | 7.72 | kJ/mol | 127.96 | Enthalp... |
| ΔvapH | 7.50 | kJ/mol | 130.00 | NIST |
| ΔvapH | 8.40 | kJ/mol | 132.00 | NIST |
| ΔvapH | 7.62 | kJ/mol | 133.15 | Enthalp... |
| ΔvapH | 8.10 | kJ/mol | 137.00 | NIST |
| ΔvapH | 8.50 | kJ/mol | 140.50 | NIST |
| ΔvapH | 8.60 | kJ/mol | 145.00 | NIST |
| ΔvapH | 8.50 | kJ/mol | 149.00 | NIST |
| ΔvapH | 5.90 | kJ/mol | 160.00 | NIST |
| ΔvapH | 8.70 | kJ/mol | 168.50 | NIST |
| ΔvapH | 4.00 | kJ/mol | 180.00 | NIST |
| Pvap | [19.65; 3170.00] | kPa | [94.93; 178.93] | |
|
T(K) Vapor pressure (kPa) 0 500 1000 1500 2000 2500 3000 100 150 | ||||
| Pvap | 19.65 | kPa | 94.93 | Investi... |
| Pvap | 34.27 | kPa | 99.97 | Investi... |
| Pvap | 55.54 | kPa | 104.84 | Investi... |
| Pvap | 87.16 | kPa | 109.87 | Investi... |
| Pvap | 88.20 | kPa | 110.01 | Vapor-L... |
| Pvap | 112.74 | kPa | 112.98 | Vapor-L... |
| Pvap | 132.11 | kPa | 114.99 | Vapor-L... |
| Pvap | 134.08 | kPa | 115.07 | Vapor-L... |
| Pvap | 153.92 | kPa | 117.00 | Vapor-L... |
| Pvap | 178.11 | kPa | 118.99 | Vapor-L... |
| Pvap | 190.62 | kPa | 119.94 | Investi... |
| Pvap | 194.65 | kPa | 120.11 | Vapor-L... |
| Pvap | 204.78 | kPa | 120.96 | Vapor-L... |
| Pvap | 235.61 | kPa | 123.01 | Vapor-L... |
| Pvap | 267.36 | kPa | 124.92 | Investi... |
| Pvap | 286.85 | kPa | 126.01 | Vapor L... |
| Pvap | 324.94 | kPa | 127.99 | Vapor L... |
| Pvap | 364.90 | kPa | 129.89 | Investi... |
| Pvap | 367.76 | kPa | 130.02 | Vapor L... |
| Pvap | 374.77 | kPa | 130.15 | Vapor-L... |
| Pvap | 413.65 | kPa | 132.01 | Vapor L... |
| Pvap | 463.11 | kPa | 133.98 | Vapor L... |
| Pvap | 486.82 | kPa | 134.87 | Investi... |
| Pvap | 496.11 | kPa | 135.09 | Vapor-L... |
| Pvap | 517.90 | kPa | 135.99 | Vapor L... |
| Pvap | 576.38 | kPa | 137.97 | Vapor L... |
| Pvap | 641.51 | kPa | 140.01 | Vapor L... |
| Pvap | 1567.00 | kPa | 159.61 | Vapor-L... |
| Pvap | 1695.00 | kPa | 161.58 | Vapor-L... |
| Pvap | 2276.00 | kPa | 169.38 | Vapor-L... |
| Pvap | 2672.00 | kPa | 173.90 | Vapor-L... |
| Pvap | 3170.00 | kPa | 178.93 | Vapor-L... |
| ρl | 425.00 | kg/m3 | 112.00 | KDB |
| γ | 0.02 | N/m | 100.00 | KDB |
| ΔvapS | 85.58 | J/mol×K | 99.54 | NIST |
Datasets
- Molar heat capacity at constant pressure, J/K/mol (1)
- Viscosity, Pa*s (1)
- Mass density, kg/m3 (2)
- Speed of sound, m/s (1)
Molar heat capacity at constant pressure, J/K/mol
| Fixed | Measured | |
|---|---|---|
| Temperature, K - Liquid | Pressure, kPa - Liquid | Molar heat capacity at constant pressure, J/K/mol - Liquid |
| 108.15 | 4230.00 | 54.40 |
| 108.15 | 6300.00 | 54.11 |
| 118.15 | 4270.00 | 55.54 |
| 118.15 | 6350.00 | 55.11 |
| 128.15 | 4330.00 | 57.03 |
| 128.15 | 6400.00 | 56.66 |
| 143.15 | 4350.00 | 59.37 |
| 153.15 | 4340.00 | 63.24 |
| 153.15 | 6340.00 | 60.98 |
| 163.15 | 4390.00 | 67.70 |
| 163.15 | 6470.00 | 64.51 |
| 173.15 | 4380.00 | 77.87 |
| 173.15 | 6450.00 | 70.97 |
| Reference | ||
Viscosity, Pa*s
| Fixed | Measured | |
|---|---|---|
| Mass density, kg/m3 - Gas | Temperature, K - Gas | Viscosity, Pa*s - Gas |
| 0.3440 | 291.93 | 0.0000 |
| 0.3440 | 292.96 | 0.0000 |
| 0.3440 | 297.08 | 0.0000 |
| 0.3440 | 323.86 | 0.0000 |
| 0.3440 | 350.55 | 0.0000 |
| 0.3440 | 378.15 | 0.0000 |
| 0.3440 | 405.88 | 0.0000 |
| 0.3440 | 433.83 | 0.0000 |
| 0.3440 | 461.85 | 0.0000 |
| 0.3440 | 490.13 | 0.0000 |
| 0.3440 | 518.83 | 0.0000 |
| 0.3440 | 542.60 | 0.0000 |
| 0.3440 | 565.38 | 0.0000 |
| 0.3440 | 594.08 | 0.0000 |
| 0.3440 | 623.33 | 0.0000 |
| 0.3440 | 652.41 | 0.0000 |
| 0.3440 | 682.14 | 0.0000 |
| 0.4980 | 289.05 | 0.0000 |
| 0.4980 | 290.41 | 0.0000 |
| 0.4980 | 296.43 | 0.0000 |
| 0.4980 | 296.57 | 0.0000 |
| 0.4980 | 323.43 | 0.0000 |
| 0.4980 | 350.64 | 0.0000 |
| 0.4980 | 378.25 | 0.0000 |
| 0.4980 | 406.03 | 0.0000 |
| 0.4980 | 434.02 | 0.0000 |
| 0.4980 | 461.75 | 0.0000 |
| 0.4980 | 490.15 | 0.0000 |
| 0.4980 | 518.80 | 0.0000 |
| 0.4980 | 542.36 | 0.0000 |
| 0.4980 | 565.10 | 0.0000 |
| 0.4980 | 593.84 | 0.0000 |
| 0.4980 | 623.05 | 0.0000 |
| 0.4980 | 652.07 | 0.0000 |
| 0.4980 | 681.93 | 0.0000 |
| Reference | ||
Mass density, kg/m3
| Fixed | Measured | |
|---|---|---|
| Pressure, kPa - Liquid | Temperature, K - Liquid | Mass density, kg/m3 - Liquid |
| 185.50 | 105.00 | 432.098 |
| 478.95 | 105.00 | 432.339 |
| 973.88 | 105.00 | 432.743 |
| 1970.76 | 105.00 | 433.554 |
| 5044.56 | 105.00 | 435.981 |
| 479.33 | 115.00 | 417.827 |
| 973.28 | 115.00 | 418.319 |
| 1969.98 | 115.00 | 419.298 |
| 477.88 | 125.00 | 402.38 |
| 972.71 | 125.00 | 402.99 |
| 1970.40 | 125.00 | 404.194 |
| 5112.40 | 125.00 | 407.803 |
| 783.75 | 135.00 | 386.1 |
| 1001.19 | 135.00 | 386.441 |
| 2014.28 | 135.00 | 387.992 |
| 5208.85 | 135.00 | 392.557 |
| Reference | ||
| Fixed | Measured | |
|---|---|---|
| Temperature, K - Fluid (supercritical or subcritical phases) | Pressure, kPa - Fluid (supercritical or subcritical phases) | Mass density, kg/m3 - Fluid (supercritical or subcritical phases) |
| 298.14 | 29958.00 | 212.508 |
| 298.14 | 124934.00 | 363.139 |
| 298.14 | 159617.00 | 385.731 |
| 298.14 | 49959.00 | 273.189 |
| 298.14 | 150062.00 | 380.039 |
| 298.14 | 170054.00 | 391.531 |
| 298.14 | 188059.00 | 400.837 |
| 298.14 | 185333.00 | 399.489 |
| 298.14 | 35056.00 | 232.422 |
| 298.14 | 99874.00 | 342.269 |
| 298.14 | 20012.00 | 157.172 |
| 298.14 | 186931.00 | 400.257 |
| 298.15 | 10010.00 | 76.08 |
| 298.15 | 79980.00 | 321.098 |
| 298.16 | 1012.00 | 6.665 |
| 298.16 | 66961.00 | 303.605 |
| 298.19 | 5009.00 | 35.316 |
| 298.25 | 14994.00 | 118.506 |
| 305.23 | 149862.00 | 376.248 |
| 305.23 | 79855.00 | 316.163 |
| 305.23 | 49968.00 | 267.232 |
| 305.23 | 9993.00 | 72.932 |
| 305.23 | 60012.00 | 287.019 |
| 305.23 | 124930.00 | 359.159 |
| 305.23 | 99904.00 | 337.939 |
| 305.24 | 6897.00 | 48.435 |
| 305.24 | 5001.00 | 34.173 |
| 305.24 | 1002.00 | 6.404 |
| 305.24 | 29976.00 | 205.443 |
| 305.24 | 34563.00 | 223.779 |
| 305.24 | 69988.00 | 302.917 |
| 305.24 | 15006.00 | 113.44 |
| 305.24 | 2000.00 | 13.031 |
| 305.24 | 20696.00 | 155.024 |
| 338.04 | 6905.00 | 42.093 |
| 338.05 | 34473.00 | 195.446 |
| 338.05 | 5000.00 | 29.983 |
| 338.06 | 70001.00 | 280.639 |
| 338.07 | 149542.00 | 359.787 |
| 338.07 | 124895.00 | 341.685 |
| 338.08 | 15026.00 | 95.246 |
| 338.08 | 59971.00 | 263.229 |
| 338.08 | 30005.00 | 177.38 |
| 338.08 | 2004.00 | 11.667 |
| 338.08 | 20687.00 | 130.132 |
| 338.08 | 1004.00 | 5.782 |
| 338.08 | 50031.00 | 241.969 |
| 338.09 | 164905.00 | 369.665 |
| 338.10 | 9969.00 | 62.054 |
| 338.10 | 80310.00 | 295.61 |
| 338.11 | 99908.00 | 318.744 |
| 338.12 | 179829.00 | 378.164 |
| 399.94 | 89964.00 | 274.637 |
| 399.97 | 59973.00 | 225.951 |
| 399.98 | 1002.00 | 4.86 |
| 399.98 | 30014.00 | 141.158 |
| 399.99 | 15027.00 | 74.789 |
| 400.00 | 50037.00 | 203.469 |
| 400.00 | 79920.00 | 260.652 |
| 400.01 | 6915.00 | 34.195 |
| 400.01 | 2001.00 | 9.744 |
| 400.01 | 10002.00 | 49.746 |
| 400.02 | 124882.00 | 312.062 |
| 400.02 | 149627.00 | 332.136 |
| 400.02 | 20675.00 | 101.642 |
| 400.03 | 13795.00 | 68.723 |
| 400.04 | 34510.00 | 157.64 |
| 400.04 | 69978.00 | 244.725 |
| 400.07 | 5005.00 | 24.61 |
| 400.09 | 99984.00 | 286.8 |
| 450.00 | 2009.00 | 8.61 |
| 450.01 | 20697.00 | 87.478 |
| 450.01 | 99932.00 | 264.608 |
| 450.02 | 119918.00 | 286.376 |
| 450.02 | 89981.00 | 251.923 |
| 450.03 | 59975.00 | 202.137 |
| 450.03 | 139476.00 | 304.187 |
| 450.05 | 30002.00 | 121.791 |
| 450.06 | 50036.00 | 179.955 |
| 450.06 | 69966.00 | 221.073 |
| 450.08 | 80008.00 | 237.571 |
| 450.09 | 6886.00 | 29.741 |
| 450.12 | 34492.00 | 136.647 |
| Reference | ||
Speed of sound, m/s
Operational condition: Frequency, MHz = 0.005 (Gas)
| Fixed | Measured | |
|---|---|---|
| Temperature, K - Gas | Pressure, kPa - Gas | Speed of sound, m/s - Gas |
| 280.01 | 500.03 | 434.024 |
| 280.01 | 500.03 | 434.026 |
| 280.01 | 500.04 | 434.027 |
| 280.01 | 500.18 | 434.032 |
| 280.02 | 500.18 | 434.03 |
| 280.02 | 500.19 | 434.03 |
| 280.02 | 500.19 | 434.032 |
| 280.02 | 500.04 | 434.027 |
| 280.02 | 500.03 | 434.027 |
| 280.02 | 500.04 | 434.03 |
| 280.02 | 500.04 | 434.034 |
| 280.02 | 500.19 | 434.034 |
| 280.02 | 500.04 | 434.018 |
| 280.02 | 500.19 | 434.027 |
| 280.02 | 500.03 | 434.028 |
| 293.27 | 394.31 | 443.975 |
| 293.27 | 394.32 | 443.969 |
| 293.27 | 394.32 | 443.97 |
| 293.27 | 394.32 | 443.971 |
| 293.27 | 394.31 | 443.973 |
| 293.27 | 394.32 | 443.973 |
| 293.27 | 394.31 | 443.973 |
| 293.28 | 394.32 | 443.967 |
| 293.28 | 394.32 | 443.969 |
| 293.28 | 394.32 | 443.97 |
| 293.28 | 394.32 | 443.974 |
| 293.28 | 394.32 | 443.973 |
| 293.28 | 394.35 | 443.976 |
| 293.28 | 394.32 | 443.973 |
| 293.28 | 394.33 | 443.975 |
| 293.28 | 394.35 | 443.977 |
| 293.28 | 394.33 | 443.979 |
| 293.28 | 394.34 | 443.977 |
| 293.28 | 394.33 | 443.974 |
| 293.28 | 394.34 | 443.979 |
| 293.29 | 394.36 | 443.976 |
| 293.29 | 394.36 | 443.972 |
| 319.52 | 429.90 | 462.063 |
| 319.53 | 429.91 | 462.063 |
| 319.55 | 428.61 | 461.973 |
| 319.55 | 428.61 | 461.973 |
| 319.87 | 471.86 | 462.118 |
| 319.87 | 471.87 | 462.121 |
| 319.88 | 471.88 | 462.12 |
| 319.88 | 471.91 | 462.132 |
| 319.89 | 471.91 | 462.129 |
| 319.89 | 471.91 | 462.133 |
| 319.89 | 471.92 | 462.129 |
| 319.89 | 471.93 | 462.125 |
| 319.89 | 471.93 | 462.13 |
| 319.94 | 430.41 | 462.324 |
| 319.94 | 430.42 | 462.32 |
| 319.95 | 429.13 | 462.235 |
| 319.96 | 429.13 | 462.236 |
| 339.35 | 456.00 | 474.758 |
| 339.35 | 456.00 | 474.763 |
| 339.88 | 456.67 | 475.085 |
| 339.89 | 456.67 | 475.079 |
| 359.27 | 481.98 | 486.936 |
| 359.28 | 481.99 | 486.939 |
| 359.28 | 481.81 | 486.759 |
| 359.28 | 480.58 | 486.844 |
| 359.28 | 481.81 | 486.762 |
| 359.29 | 480.58 | 486.847 |
| 359.85 | 482.79 | 487.307 |
| 359.86 | 482.79 | 487.315 |
| 359.86 | 482.59 | 487.148 |
| 359.87 | 482.60 | 487.149 |
| 360.03 | 529.63 | 487.351 |
| 360.03 | 529.64 | 487.351 |
| 360.03 | 529.64 | 487.345 |
| 360.03 | 529.66 | 487.346 |
| 360.04 | 529.67 | 487.332 |
| 360.04 | 529.67 | 487.335 |
| 360.04 | 529.66 | 487.34 |
| 360.04 | 529.66 | 487.341 |
| 379.20 | 507.87 | 498.656 |
| 379.20 | 507.87 | 498.659 |
| 379.78 | 508.56 | 498.971 |
| 379.78 | 508.56 | 498.975 |
| 399.24 | 533.63 | 510.014 |
| 399.24 | 533.63 | 510.016 |
| 399.82 | 534.48 | 510.391 |
| 399.82 | 534.48 | 510.388 |
| 399.99 | 471.94 | 510.49 |
| 399.99 | 471.94 | 510.498 |
| 399.99 | 471.95 | 510.472 |
| 399.99 | 471.98 | 510.469 |
| 399.99 | 471.96 | 510.471 |
| 399.99 | 471.97 | 510.474 |
| 399.99 | 471.95 | 510.477 |
| 399.99 | 471.94 | 510.491 |
| 400.00 | 471.96 | 510.465 |
| 419.54 | 559.42 | 521.018 |
| 419.55 | 559.43 | 521.03 |
| 419.56 | 559.63 | 521.204 |
| 419.56 | 559.63 | 521.203 |
| 419.99 | 560.00 | 521.281 |
| 420.00 | 560.01 | 521.291 |
| 420.00 | 560.01 | 521.28 |
| 420.00 | 560.01 | 521.285 |
| 420.02 | 560.19 | 521.466 |
| 420.02 | 560.20 | 521.455 |
| 439.94 | 496.88 | 532.013 |
| 439.94 | 496.88 | 532.015 |
| 439.94 | 496.88 | 532.015 |
| 439.94 | 496.88 | 532.017 |
| 439.94 | 496.87 | 532.023 |
| 439.94 | 496.88 | 532.026 |
| 439.94 | 496.86 | 532.021 |
| 439.94 | 496.86 | 532.033 |
| 439.94 | 496.85 | 532.026 |
| 449.69 | 597.89 | 537.244 |
| 449.69 | 597.90 | 537.24 |
| 449.69 | 597.90 | 537.242 |
| 449.69 | 597.90 | 537.232 |
| 449.69 | 597.90 | 537.242 |
| 449.69 | 597.90 | 537.227 |
| 449.69 | 597.90 | 537.246 |
| 449.70 | 597.90 | 537.23 |
| 449.70 | 597.90 | 537.24 |
| 449.70 | 597.90 | 537.228 |
| 449.70 | 597.89 | 537.223 |
| 449.70 | 597.88 | 537.215 |
| 449.70 | 597.89 | 537.229 |
| 449.71 | 597.88 | 537.216 |
| 449.71 | 597.89 | 537.221 |
| 449.71 | 597.89 | 537.218 |
| 449.71 | 597.88 | 537.209 |
| 449.71 | 597.89 | 537.209 |
| 449.71 | 597.89 | 537.213 |
| 449.71 | 597.89 | 537.216 |
| 449.72 | 597.89 | 537.217 |
| 449.72 | 597.89 | 537.204 |
| 449.72 | 597.88 | 537.197 |
| 449.72 | 597.89 | 537.209 |
| 449.72 | 597.89 | 537.199 |
| 449.73 | 597.87 | 537.202 |
| 449.73 | 597.87 | 537.192 |
| 449.73 | 597.87 | 537.194 |
| 449.73 | 597.86 | 537.191 |
| 449.74 | 597.87 | 537.201 |
| 449.74 | 597.86 | 537.19 |
| 449.74 | 597.87 | 537.196 |
| 449.77 | 597.88 | 537.202 |
| 449.77 | 597.88 | 537.201 |
| 449.78 | 597.90 | 537.204 |
| 449.79 | 597.90 | 537.196 |
| 479.91 | 495.54 | 552.379 |
| 479.92 | 495.57 | 552.381 |
| 479.92 | 495.54 | 552.386 |
| 479.92 | 495.55 | 552.39 |
| 479.92 | 495.52 | 552.398 |
| 479.92 | 539.80 | 552.375 |
| 479.92 | 495.57 | 552.387 |
| 479.92 | 539.80 | 552.39 |
| 479.92 | 495.51 | 552.397 |
| 479.92 | 539.81 | 552.388 |
| 479.92 | 495.51 | 552.391 |
| Reference | ||
Correlations
| Property | Value | Unit | Temperature (K) | Source |
|---|---|---|---|---|
| Pvap | [1.33; 4590.83] | kPa | [76.30; 190.56] |
The Yaw...
|
| Equation | ln(Pvp) = A + B/(T + C) | |||
| Coefficient A | 1.37434e+01 | |||
| Coefficient B | -1.00267e+03 | |||
| Coefficient C | -1.78900e+00 | |||
| Temperature range, min. | 76.30 | |||
| Temperature range, max. | 190.56 | |||
|
T(K) Vapor pressure (kPa) 0 1000 2000 3000 4000 5000 100 150 | ||||
| Pvap | 1.33 | kPa | 76.30 | Calculated Property |
| Pvap | 9.45 | kPa | 89.00 | Calculated Property |
| Pvap | 40.73 | kPa | 101.69 | Calculated Property |
| Pvap | 126.28 | kPa | 114.39 | Calculated Property |
| Pvap | 311.31 | kPa | 127.08 | Calculated Property |
| Pvap | 650.07 | kPa | 139.78 | Calculated Property |
| Pvap | 1199.04 | kPa | 152.47 | Calculated Property |
| Pvap | 2010.90 | kPa | 165.17 | Calculated Property |
| Pvap | 3130.16 | kPa | 177.86 | Calculated Property |
| Pvap | 4590.83 | kPa | 190.56 | Calculated Property |
Similar Compounds
Find more compounds similar to Methane.
Mixtures
- Carbon dioxide + Methane
- Methane + Ethanol
- Heptadecane + Methane
- Dimethyl sulfide + Methane
- Methane + Ethane, (methylthio)-
- Methanethiol + Methane
- Methane + Ethanethiol
- Methane + Octadecane
- Methane + Tetradecane
- Nitrogen + Methane
- Isopropyl Alcohol + Methane
- Methane + 1-Butanol
- Methane + 1,3-Dioxolan-2-one
- Propylene Carbonate + Methane
- Methane + Carbonic acid, dimethyl ester
- Methane + Carbonic acid, ethyl-, methyl ester
- Diethyl carbonate + Methane
- Methane + Carbonic acid, dimethyl ester + 1,3-Dioxolan-2-one
- Propane + Methane
- Methane + Perfluorooctane
Find more mixtures with Methane.
Sources
- Crippen Method
- Thermal Conductivity of Carbon Dioxide Methane Mixtures at Temperatures Between 300 and 425K and at Pressures up to 12 MPa
- Measurements of the Calorific Value of Methane with the New GERG Reference Calorimeter
- The Experimental Study of Temperature Dependence of Binary Diffusion Coefficients of Gases at Different Pressures
- Binary Diffusion Coefficients for Gas Mixtures of Propane with Methane and Carbon Dioxide Measured in a Loschmidt Cell Combined with Holographic Interferometry
- Liquid-liquid equilibrium data of water with neohexane, methylcyclohexane, tert-butyl methyl ether, n-heptane and vapor -liquid-liquid equilibrium with methane
- Viscosity, density, interfacial tension and compositional data for near critical mixtures of methane + butane and methane + decane systems at 310.95K
- Establishing benchmarks for the Second Industrial Fluids Simulation Challenge
- Vapor liquid equilibrium of methane and methane + nitrogen and an equimolar hexane + decane mixture under isothermal conditions
- Measurement and modeling of hydrocarbon dew points for five synthetic natural gas mixtures
- Low-pressure solubilities and thermodynamics of solvation of eight gases in 1-butyl-3-methylimidazolium hexafluorophosphate
- High-pressure vapor liquid equilibria of systems containing ethylene glycol, water and methane Experimental measurements and modeling
- High pressure phase equilibria in methane +waxy systems 1. Methane + heptadecane
- Measurement of VLE (TPx or TPxy data) for hydrogen sulfide + (dimethylsulfide or ethylmethylsulfide or carbon disulfide) and methane solubilities in (dimethylsulfide or ethylmethylsulfide or methylmercaptan or ethylmercaptan)
- Measurement of the triethylene glycol solubility in supercritical methane at pressures up to 9MPa
- Methane and carbon dioxide solubility in 1,2-propylene glycol at temperatures ranging from 303 to 423 K and pressures up to 12 MPa
- High pressure phase behaviour of methane in 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide
- Gas gas experimental interfacial tension measurement
- Experimental data on binary and ternary mixtures of perfluoro-1,3-dimethylcyclohexane with normal alkanes at 373.15 K
- Capillary constant and surface tension of methane-nitrogen solutions: 1. Experiment
- Measurement and equation of state prediction of vapor-liquid equilibrium and physical properties for the system methane + n-octadecane
- Solubilities of several non-polar gases in mixtures water + 2,2,2-trifluoroethanol at 298.15 K and 101.33 kPa
- Surface tensions of aqueous solutions of lithium dodecyl sulfate, sodium oleate, and dodecylbenzene sulfonic acid in contact with methane under hydrate-forming conditions
- Vapor liquid equilibrium measurement and thermodynamic modeling of binary systems (methane + n-tetradecane)
- Development and testing of a new apparatus for the measurement of high-pressure low-temperature phase equilibria
- Experimental and thermodynamic modeling study on (vapor + liquid) equilibria and physical properties of ternary systems (methane + n-decane + n-tetradecane)
- Surface tension of ethane-methane solutions: 1. Experiment and thermodynamic analysis of the results
- High pressure phase equilibria of binary mixtures of light hydrocarbons in the ionic liquid 1-hexyl-3-methylimidazoliumtetracyanoborate
- In situ Raman spectroscopic study of diffusion coefficients of methane in liquid water under high pressure and wide temperatures
- Effect of impurities in captured CO2 on liquid-vapor equilibrium
- Isothermal vapor-liquid equilibrium in CH4/H2/N2 system at a cryogenic temperature range from 100.0 K to 125.0 K
- Solubility of carbon dioxide, nitrous oxide and methane in ionic liquids at pressures close to atmospheric
- Solubility of CO2/CH4 gas mixtures in ionic liquids
- Phase equilibrium data for the hydrogen sulphide + methane system at temperatures from 186 to 313 K and pressures up to about 14 MPa
- An experimental investigation of the phase equilibrium of the binary system (methane + water) at low temperatures: Solubility of methane in water and three-phase (vapour + liquid + hydrate) equilibrium
- Physical data for a process to separate krypton from air by selective absorption in an ionic liquid
- Measurement of vapor-liquid-liquid phase equilibrium - Equipment and results
- Effects of association with impurities in ammonia purification
- Accurate density measurements on a binary mixture (carbon dioxide + methane) at the vicinity of the critical point in the supercritical state by a single-sinker densimeter
- Measurement and modelling of interfacial tension in methane/water and methane/brine systems at reservoir conditions
- Interfacial tension measurements in water-methane system at temperatures from 278.15 K to 298.15 K and pressures up to 10 MPa
- Anomalous nucleation near a fluid phase boundary created by a rapid heat pulse
- Separation of methane/ethylene gas mixtures efficiently by using ZIF- 67/water-ethylene glycol slurry
- High pressure vapor-liquid equilibria for binary methane and protic ionic liquid based on propionate anions
- Experimental and modelling study of the densities of the hydrogen sulphide + methane mixtures at 253, 273 and 293 K and pressures up to 30 MPa.
- Density and phase equilibrium of the binary system methane + n-decane under high temperatures and pressures
- An experimental investigation on the solubility of methane in 1-octanol and n-dodecane at ambient temperatures
- Surface tension, density and composition in the methane-pentane system at high pressure
- Low viscosity protic ionic liquid for CO2/CH4 separation: Thermophysical and high-pressure phase equilibria for diethylammonium butanoate
- Vapor - liquid equilibrium of the carbon dioxide/methane mixture at three isotherms
- Experimental P(rho)T-x measurements of liquid methane-ethane-nitrogen mixtures
- Guaiacol and its mixtures: new data and predictive models Part 1: Phase equilibrium
- Interface light-scattering on a methane-decane system in the near-critical region at 37.8 deg.C (100 deg.F)
- High-pressure experimental vapour-liquid-liquid equilibrium measurements and modelling for natural gas processing: Equipment validation, and the system CH4 + nC6H14 + H2O
- A re-entrant resonator for the measurement of phase boundaries: dew points for {0.4026CH4 + 0.5974C3H8}
- Solubility of carbon dioxide, ethane, methane, oxygen, nitrogen, hydrogen, argon, and carbon monoxide in 1-butyl-3-methylimidazolium tetrafluoroborate between temperatures 283 K and 343 K and at pressures close to atmospheric
- Measurement of the (pressure, density, temperature) relation of two (methane + nitrogen) gas mixtures at temperatures between 240 and 400 K and pressures up to 20 MPa using an accurate single-sinker densimeter
- Speeds of sound in {(1 - x)CH4 + xN2} with x = (0.10001, 0.19999, and 0.5422) at temperatures between 170 K and 400 K and pressures up to 30 MPa
- Solubility of methane in water and in a medium for the coltivation of methanotrophs bacteria
- Solubility of methane and carbon dioxide in ethylene glycol at pressures up to 14 MPa and temperatures ranging from (303 to 423) K
- Phase equilibrium measurements and thermodynamic modeling of methane alcohol systems at ambient temperature
- Solubility of gases in 1-alkyl-3methylimidazolium alkyl sulfate ionic liquids: Experimental determination and modeling
- Measurement of the (pressure, density, temperature) relation of a (methane + nitrogen) gaseous mixture using an accurate single-sinker densimeter
- Experimental and modeling investigations of solubility and saturated liquid densities and viscosities for binary systems (methane +, ethane +, and carbon dioxide + 2-propanol)
- Solubility of carbon dioxide, methane, and ethane in 1-butanol and saturated liquid densities and viscosities
- Low pressure methane solubility in lithium-ion batteries based solvents and electrolytes as a function of temperature. Measurement and prediction
- Phase equilibrium measurements of (methane + benzene) and (methane + methylbenzene) at temperatures from (188 to 348) K and pressures to 13 MPa
- Viscosity of {xCO2 + (1-x)CH4} with x = 0.5174 for temperatures between (229 and 348) K and pressures between (1 and 32) MPa
- Spherical resonator for vapor-phase speed of sound and measurements of 1,1,1,2,2,3,3-heptafluoro-3-methoxypropane (RE347mcc) and trans-1,3,3,3-tetrafluoropropene [R1234ze(E)]
- Development of a special single-sinker densimeter for cryogenic liquid mixtures and first results for a liquefied natural gas (LNG)
- Experimental determination of (p,.rho.,T) data for binary mixtures of methane and helium
- Measurement and modelling of high pressure density and interfacial tension of (gas + n-alkane) binary mixtures
- Application of a two-sinker densimeter for phase-equilibrium measurements: A new technique for the detection of dew points and measurements on the (methane + propane) system
- Solubility of methane in n-hexane and a petroleum benzine at ambient temperatures
- Accurate experimental (p, .rho., T) values and virial coefficients for the (methane and helium) binary system
- Density characteristics of CO2-CH4 binary mixtures at temperatures from (300 to 308.15) K and pressures from (2 to 18) MPa
- Surface tension and critical point measurements of methane + propane mixtures
- Solubility of carbon dioxide and methane in 1-hexyl-3-methylimidazolium nitrate ionic liquid, experimental and thermodynamic modeling
- Solubility of gases in fluoroorganic alcohols. Part III. Solubilities of several non-polar gases in water + 1,1,1,3,3,3-hexafluoropropan-2-ol at 298.15 K and 101.33 kPa
- Solubilities of gases in cycloethers. The solubility of 13 nonpolar gases in 2,5-dimethyltetrahydrofuran at 273.15 to 303.15 K and 101.32 kPa
- A novel technique based in a cylindrical microwave resonator for high pressure phase equilibrium determination
- Speeds of sound for (CH4 + He) mixtures from p = (0.5 to 20) MPa at T = (273.16 to 375) K
- Measurement and Calculation of CO2 Frost Points in CH4 + CO2/CH4 + CO2 + N2/CH4 + CO2 + C2H6 Mixtures at Low Temperatures
- Phase Equilibrium Measurements and Modeling of 1-Propanethiol +1-Butanethiol + CH4 in Methane Ternary System at 303, 336, and 368 K and Pressure Up to 9 MPa
- Reference Quality Vapor-Liquid Equilibrium Data for the Binary Systems Methane + Ethane, + Propane, + Butane and, + 2-Methylpropane, at Temperatures from (203 to 273) K and Pressures to 9 MPa
- Viscosity and dew point measurements of {xCH4 + (1- x)C4H10} for x = 0.9484 with a vibrating-wire viscometer.
- Experimental Measurement and Modeling of the Solubility of Methane in Methanol and Ethanol
- Density Measurements of Methane + Propane Mixtures at Temperatures between (256 and 422) K and Pressures from (24 to 35) MPa
- Accurate p-.rho.-T Data for a Synthetic Residual Natural Gas Mixture (0.95 CH4 + 0.04 C2H6 + 0.01 C3H8) at Temperatures between (135 and 500) K at Pressures to 200 MPa
- Density Measurements for Ethane, Carbon Dioxide, and Methane + Nitrogen Mixtures from 300 to 470 K up to 137 MPa Using a Vibrating Tube Densimeter
- Solubility of Mercury in Liquid Hydrocarbons and Hydrocarbon Mixtures
- Solubility Measurement and Modeling of Methane in Methanol and Ethanol Aqueous Solutions
- Interfacial Tension and Related Properties of Ionic Liquids in CH4 and CO2 at Elevated Pressures: Experimental Data and Molecular Dynamics Simulation
- Enthalpy of Vaporization Measurements of Liquid Methane, Ethane, and Methane + Ethane by Differential Scanning Calorimetry at Low Temperatures and High Pressures
- Water Solubility at Saturation for CO2 CH4 Mixtures at 323.2 K and 9.000 MPa
- Binary Vapor-Liquid Equilibrium Data for Perfluorooctane with Light Gases (Oxygen, Nitrogen, and Methane)
- Solubility of Methane and Carbon Dioxide in the Aqueous Phase of the Ternary (Methane + Carbon Dioxide + Water) Mixture: Experimental Measurements and Molecular Dynamics Simulations
- Density and Compressibility of Multicomponent n-Alkane Mixtures up to 463 K and 140 MPa
- Accurate Experimental (p, rho, and T) Data for the Introduction of Hydrogen into the Natural Gas Grid (II): Thermodynamic Characterization of the Methane-Hydrogen Binary System from 240 to 350 K and Pressures up to 20 MPa
- Ternary Vapor-Liquid Equilibrium Measurements and Modeling of Ethylene Glycol (1) + Water (2) + Methane (3) Systems at 6 and 12.5 MPa
- Investigation on Vapor-Liquid Equilibrium of (Argon + Methane) at the Temperature Range of (95 to 135) K
- Density Characteristics of the CO2-CH4 Binary System: Experimental Data at 313-353 K and 3-18 MPa and Modeling from the PC-SAFT EoS
- Measurement and Prediction of Hydrocarbon Dew Points of Synthetic Natural Gas Mixtures
- Density Data of Two (H2 + CO2) Mixtures and a (H2 + CO2 + CH4) Mixture by a Modified Burnett Method at Temperature 673 K and Pressures up to 25 MPa
- Solubilities of Nonpolar Gases in Triethylene Glycol Dimethyl Ether, Tetraethylene Glycol Dimethyl Ether, Dimethyl Carbonate, and Diethyl Carbonate at 298.15 K and 101.33 kPa Partial Pressure of Gas
- Determination and Study on the Solubility of Methane in Heptane + Cyclohexane and Heptane + Ethanol at High Pressures
- Experimental Data on Binary and Ternary Mixtures of Perfluoromethylcyclohexane with Methane and n-Decane at 373.15 K
- Measurements of the Speed of Sound for Mixtures of Methane + Butane with a Particular Focus on the Critical State
- Vibrating Wire Viscometer with Wire Diameters of (0.05 and 0.15) mm: Results for Methylbenzene and Two Fluids with Nominal Viscosities at T = 298 K and p = 0.01 MPa of (14 and 232) mPa*s at Temperatures between (298 and 373) K and Pressures below 40 MPa
- Surface Tensions of Aqueous Solutions of Sodium Alkyl Sulfates in Contact with Methane under Hydrate-Forming Conditions
- Solubility Study of Methane and Ethane In Promising Physical Solvents for Natural Gas Sweetening Operations
- Solubilities of Gases in 1,1,3,3-Tetramethylguanidium Lactate at Elevated Pressures
- Determination and Study of the Solubility of Methane in Mixtures of Methanol plus Various Hydrocarbons at High Pressures
- Solubility of Methane in Methyldiethanolamine
- Measurement of Carbon Dioxide Freezing in Mixtures of Methane, Ethane, and Nitrogen in the Solid-Vapor Equilibrium Region
- Vapor-Liquid Equilibrium Data of the Methane + Tetrafluoromethane System at Temperatures from (159.61 to 178.93) K
- Accurate P-rho-T Data and Phase Boundary Determination for a Synthetic Residual Natural Gas Mixture
- Influence of Compressed Carbon Dioxide on the Capillarity of the Gas-Crude Oil-Reservoir Water System
- Equilibrium Phase Densities, Interfacial Tensions for the Ethane + n-Pentane System at 294.15 K
- Vapor-Liquid Equilibria Measurements of the Methane + Pentane and Methane + Hexane Systems at Temperatures from (173 to 330) K and Pressures to 14 MPa
- Experimental Determination of the Solubilities of CO2 and CH4 in Diethyl Methylphosphonate
- Measurement and Modeling of CO2 Frost Points in the CO2 Methane Systems
- Equilibrium Phase Densities, Vapor Phase Compositions, and Interfacial Tensions for the Methane + Ethane + n-Pentane System at 294.15 K
- Reference Viscosities of Gaseous Methane and Hydrogen Sulfide at Low Density in the Temperature Range from (292 to 682) K
- Interfacial Tension between Methane and Octane at Elevated Pressure at Five Temperatures from (274.2 to 282.2) K
- Experimental Studies of Solubility of Elemental Sulfur in Methane at 363.15 K for Pressure Ranging from (4 to 25) MPa
- Phase Equilibria of Three Binary Mixtures: Methanethiol + Methane, Methanethiol + Nitrogen, and Methanethiol + Carbon Dioxide
- Vapor-Liquid Equilibrium for the Mixture Nitrogen (N2) + Methane (CH4) in the Temperature Range of (110 to 125) K
- Determination of CO2 Solubility in Saturated Liquid CH4 + N2 and CH4 + C2H6 Mixtures above Atmospheric Pressure
- Vapor Liquid Phase Equilibria and Physical Properties Measurements for Ternary Systems (Methane + Decane + Hexadecane)
- Solubility Measurements and Saturated Liquid Properties of Ternary Systems (Methane + Decane + Octadecane) at 295 K
- Isobaric Heat Capacity Measurements of Liquid Methane, Ethane, and Propane by Differential Scanning Calorimetry at High Pressures and Low Temperatures
- Vapor Liquid Equilibrium for the Mixture Methane (CH4) + Ethane (C2H6) over the Temperature Range (126.01 to 140.01) K
- Vapor Liquid Equilibrium of Methane with Water and Methanol. Measurements and Modeling
- Isobaric Heat Capacity Measurements of Liquid Methane + Propane, Methane + Butane, and a Mixed Refrigerant by Differential Scanning Calorimetry at High Pressures and Low Temperatures
- Improved Methods for Gas Mixture Viscometry Using a Vibrating Wire Clamped at Both Ends
- Isothermal Vapor Liquid Equilibrium Data and Thermodynamic Modeling for Binary Systems of Perfluorobutane (R610) + (Methane or Hydrogen Sulfide) at (293, 313, and 333) K
- PvT Measurements of trans-1,3,3,3-Tetrafluoroprop-1-ene + Methane and trans-1,3,3,3-Tetrafluoroprop-1-ene + Nitrogen Binary Pairs
- Vapor-Phase (p, n, T, x) Behavior and Virial Coefficients for the (Methane + Propane) System
- Solubility of Methane in Propylene Carbonate
- Viscosity of {xCH4 + (1-x)C3H8} with x = 0.949 for Temperatures Between (200 and 423) K and Pressures Between (10 and 31) MPa
- Experimental Study: The Impact of Dissolved Water on the Viscosity of Reservoir Fluids at HPHT Conditions
- Vapor Liquid Equilibrium Measurements and Thermodynamic Modeling of the System (Methane + Cyclohexane + Ethanol)
- High-Pressure Solubility Data of Methane in Aniline and Aqueous Aniline Systems
- Critical Temperatures and Pressures of Several Binary and Ternary Mixtures Concerning the Alkylation of 2-Methylpropane with 1-Butene in the Presence of Methane or Carbon Dioxide
- Experimental Study of Solubility of Natural Gas Components in Aqueous Solutions of Ethylene Glycol at Low-Temperature and High-Pressure Conditions
- Vapor-Liquid Equilibria and Saturated Liquid Densities in Binary Mixtures of Nitrogen, Methane, and Ethane and Their Correlation Using the VTPR and PSRK GCEOS
- Solubility of the Single Gases Methane and Xenon in the Ionic Liquid [bmim][CH3SO4]
- Experimental Measurements of Vapor Liquid Equilibria of the H2O + CO2 + CH4 Ternary System
- Solubility of Methane in an Aqueous Methyldiethanolamine Solution (Mass Fraction 50 %)
- Solubilities of Carbon Dioxide, Methane, and Ethane in Sodium Chloride Solution Containing Gas Hydrate
- Solubility of Methane in Tetrahydrofuran + Ethanol at High Pressures
- Vapor-Liquid Equilibrium Measurements and Modeling of the Propyl Mercaptan + Methane + Water System
- Accurate PVT Data for Methane from (300 to 450) K up to 180 MPa
- Experimental Measurement and Thermodynamic Modeling for the Solubility of Methane in Water and Hexadecane
- Vapour Liquid Equilibria Measurements of Methane + 2- Methylpropane (Isobutane) at Temperatures from (150 to 250) K and Pressures to 9 MPa
- KDB
- Aqueous Solubility Prediction Method
- Estimated Solubility Method
- McGowan Method
- NIST Webbook
- The Yaws Handbook of Vapor Pressure
Note: Cheméo is only indexing the data, follow the source links to retrieve the latest data. The source is also providing more information like the publication year, authors and more.
Take the time to validate and double check the source of the data.
Outlier This icon means
that the value is more than 2 standard deviations away from the
property mean.