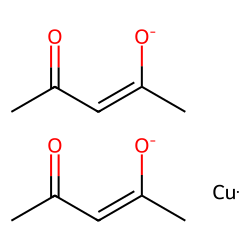
Physical Properties
| Property | Value | Unit | Source |
|---|---|---|---|
| ΔsubH° | [109.90; 154.00] | kJ/mol |
|
| ΔsubH° | 127.00 ± 1.20 | kJ/mol | NIST |
| ΔsubH° | 127.10 ± 1.20 | kJ/mol | NIST |
| ΔsubH° | 127.00 ± 1.10 | kJ/mol | NIST |
| ΔsubH° | 116.60 ± 2.00 | kJ/mol | NIST |
| ΔsubH° | 115.10 ± 2.10 | kJ/mol | NIST |
| ΔsubH° | 127.50 ± 3.20 | kJ/mol | NIST |
| ΔsubH° | Outlier 154.00 ± 22.00 | kJ/mol | NIST |
| ΔsubH° | 109.90 ± 3.40 | kJ/mol | NIST |
| IE | [7.20; 8.35] | eV |
|
| IE | 7.20 | eV | NIST |
| IE | 8.31 ± 0.05 | eV | NIST |
| IE | 7.75 ± 0.05 | eV | NIST |
| IE | 8.35 | eV | NIST |
| IE | 7.66 | eV | NIST |
| IE | 8.35 | eV | NIST |
| IE | 8.20 | eV | NIST |
| S°solid,1 bar | 372.80 | J/mol×K | NIST |
Temperature Dependent Properties
| Property | Value | Unit | Temperature (K) | Source |
|---|---|---|---|---|
| Cp,solid | 213.40 | J/mol×K | 298.00 | NIST |
| ΔsubH | [107.10; 142.60] | kJ/mol | [387.50; 492.00] | |
|
T(K) Enthalpy of sublimation at a given temperature (kJ/mol) 110 120 130 140 400 450 | ||||
| ΔsubH | 122.50 ± 1.20 | kJ/mol | 387.50 | NIST |
| ΔsubH | 122.60 ± 0.70 | kJ/mol | 387.50 | NIST |
| ΔsubH | 122.30 ± 1.10 | kJ/mol | 393.00 | NIST |
| ΔsubH | 109.00 ± 6.00 | kJ/mol | 400.00 | NIST |
| ΔsubH | 121.60 ± 1.40 | kJ/mol | 403.00 | NIST |
| ΔsubH | 120.00 | kJ/mol | 428.00 | NIST |
| ΔsubH | 142.60 ± 6.90 | kJ/mol | 471.00 | NIST |
| ΔsubH | 107.10 ± 5.70 | kJ/mol | 492.00 | NIST |
Similar Compounds
Find more compounds similar to Copper, bis(2,4-pentanedionato-O,O')-, (SP-4-1)-.
Sources
Note: Cheméo is only indexing the data, follow the source links to retrieve the latest data. The source is also providing more information like the publication year, authors and more.
Take the time to validate and double check the source of the data.
Outlier This icon means
that the value is more than 2 standard deviations away from the
property mean.