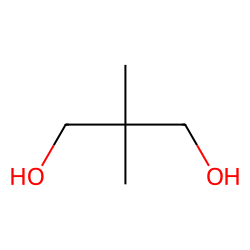
Physical Properties
Property
Value
Unit
Source
Δc H°solid -3131.30 ± 4.30
kJ/mol
NIST
Δf G° -279.58
kJ/mol
Joback Calculated Property
Δf H°gas -459.74
kJ/mol
Joback Calculated Property
Δf H°solid -551.00 ± 4.20
kJ/mol
NIST
Δfus H° 12.24
kJ/mol
Heat ca...
Δvap H° 58.79
kJ/mol
Joback Calculated Property
log 10 WS-0.20
Crippen Calculated Property
log Poct/wat -0.003
Crippen Calculated Property
McVol 93.050
ml/mol
McGowan Calculated Property
Pc 4474.22
kPa
Joback Calculated Property
Inp 895.00
NIST
Tboil [473.00; 478.95]
K
Tboil 478.95
K
Investi...
Tboil 475.65 ± 4.00
K
NIST
Tboil 473.00 ± 2.00
K
NIST
Tc 687.00
K
Vapor-l...
Tfus [398.70; 403.00]
K
Tfus 400.50 ± 1.50
K
NIST
Tfus 398.70 ± 2.00
K
NIST
Tfus 400.00 ± 3.00
K
NIST
Tfus 403.00 ± 1.50
K
NIST
Tfus 403.00 ± 3.00
K
NIST
Ttriple [314.95; 403.30]
K
Ttriple 314.95
K
Phase t...
Ttriple 403.30 ± 0.10
K
NIST
Vc 0.343
m3 /kmol
Joback Calculated Property
Temperature Dependent Properties
Property
Value
Unit
Temperature (K)
Source
Cp,gas [216.54; 259.45]
J/mol×K
[494.93; 661.34]
Cp,gas 216.54
J/mol×K
494.93
Joback Calculated Property
Cp,gas 224.63
J/mol×K
522.67
Joback Calculated Property
Cp,gas 232.33
J/mol×K
550.40
Joback Calculated Property
Cp,gas 239.64
J/mol×K
578.14
Joback Calculated Property
Cp,gas 246.59
J/mol×K
605.87
Joback Calculated Property
Cp,gas 253.18
J/mol×K
633.61
Joback Calculated Property
Cp,gas 259.45
J/mol×K
661.34
Joback Calculated Property
Cp,solid [183.18; 183.18]
J/mol×K
[298.15; 298.15]
Cp,solid 183.18
J/mol×K
298.15
NIST
Cp,solid 183.18
J/mol×K
298.15
NIST
η [0.0001025; 0.1849815]
Pa×s
[270.17; 494.93]
η 0.1849815
Pa×s
270.17
Joback Calculated Property
η 0.0247700
Pa×s
307.63
Joback Calculated Property
η 0.0051321
Pa×s
345.09
Joback Calculated Property
η 0.0014473
Pa×s
382.55
Joback Calculated Property
η 0.0005115
Pa×s
420.01
Joback Calculated Property
η 0.0002144
Pa×s
457.47
Joback Calculated Property
η 0.0001025
Pa×s
494.93
Joback Calculated Property
Δfus H [4.23; 13.80]
kJ/mol
[244.00; 403.20]
Δfus H 7.52
kJ/mol
244.00
NIST
Δfus H 13.80
kJ/mol
315.20
NIST
Δfus H 4.55
kJ/mol
401.20
NIST
Δfus H 4.23
kJ/mol
401.60
NIST
Δfus H 4.30
kJ/mol
402.50
NIST
Δfus H 4.34
kJ/mol
402.80
NIST
Δfus H 4.60
kJ/mol
403.20
NIST
Δfus H 4.60
kJ/mol
403.20
NIST
Δvap H 79.40
kJ/mol
440.00
NIST
Psub [0.01; 0.17]
kPa
[314.30; 347.20]
Psub 0.01
kPa
314.30
Vapor P...
Psub 0.01
kPa
317.30
Vapor P...
Psub 0.02
kPa
320.20
Vapor P...
Psub 0.02
kPa
323.40
Vapor P...
Psub 0.03
kPa
326.40
Vapor P...
Psub 0.04
kPa
329.40
Vapor P...
Psub 0.05
kPa
332.30
Vapor P...
Psub 0.07
kPa
335.30
Vapor P...
Psub 0.08
kPa
338.20
Vapor P...
Psub 0.11
kPa
341.20
Vapor P...
Psub 0.13
kPa
344.20
Vapor P...
Psub 0.17
kPa
347.20
Vapor P...
Pvap 101.30
kPa
478.95
Investi...
Δfus S [11.41; 43.78]
J/mol×K
[315.20; 403.20]
Δfus S 43.78
J/mol×K
315.20
NIST
Δfus S 11.41
J/mol×K
403.20
NIST
λ [0.29; 0.40]
W/m×K
[323.00; 393.00]
λ 0.40
W/m×K
323.00
Depende...
λ 0.38
W/m×K
333.00
Depende...
λ 0.36
W/m×K
343.00
Depende...
λ 0.33
W/m×K
353.00
Depende...
λ 0.32
W/m×K
363.00
Depende...
λ 0.29
W/m×K
373.00
Depende...
λ 0.30
W/m×K
383.00
Depende...
λ 0.31
W/m×K
393.00
Depende...
Correlations
Property
Value
Unit
Temperature (K)
Source
Pvap [2.90; 4249.61]
kPa
[400.00; 643.00]
KDB Vap...
Equation ln(Pvp) = A + B/T + C*ln(T) + D*T^2 Coefficient A 2.32555e+02 Coefficient B -1.93531e+04 Coefficient C -3.09511e+01 Coefficient D 1.45892e-05 Temperature range, min. 400.00 Temperature range, max. 643.00
Pvap 2.90
kPa
400.00
Calculated Property
Pvap 11.33
kPa
427.00
Calculated Property
Pvap 35.60
kPa
454.00
Calculated Property
Pvap 94.20
kPa
481.00
Calculated Property
Pvap 217.70
kPa
508.00
Calculated Property
Pvap 452.06
kPa
535.00
Calculated Property
Pvap 862.60
kPa
562.00
Calculated Property
Pvap 1540.02
kPa
589.00
Calculated Property
Pvap 2610.10
kPa
616.00
Calculated Property
Pvap 4249.61
kPa
643.00
Calculated Property
Similar Compounds
Find more compounds similar to Neopentyl glycol .
Mixtures
Sources
Note: Cheméo is only indexing the data, follow the source links to retrieve the latest data. The source is also providing more information like the publication year, authors and more.
Take the time to validate and double check the source of the data.