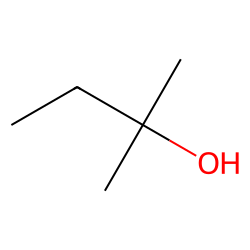
Physical Properties
Property
Value
Unit
Source
Δc H°liquid -3303.10 ± 0.46
kJ/mol
NIST
μ 1.90
debye
KDB
Δf G° -142.76
kJ/mol
Joback Calculated Property
Δf H°gas [-329.90; -329.30]
kJ/mol
Δf H°gas -329.90
kJ/mol
KDB
Δf H°gas -329.30
kJ/mol
NIST
Δf H°liquid -379.50 ± 0.54
kJ/mol
NIST
Δfus H° 0.90
kJ/mol
Heat Ca...
Δvap H° [49.20; 51.50]
kJ/mol
Δvap H° 50.17
kJ/mol
NIST
Δvap H° 51.50 ± 0.30
kJ/mol
NIST
Δvap H° 50.50
kJ/mol
NIST
Δvap H° 50.20 ± 0.30
kJ/mol
NIST
Δvap H° 50.10 ± 0.20
kJ/mol
NIST
Δvap H° 49.20
kJ/mol
NIST
IE [9.80; 10.16]
eV
IE 9.80
eV
NIST
IE 10.16 ± 0.03
eV
NIST
log 10 WS[0.08; 0.15]
log 10 WS0.08
Aq. Sol...
log 10 WS0.15
Estimat...
log Poct/wat 1.167
Crippen Calculated Property
McVol 87.180
ml/mol
McGowan Calculated Property
NFPA Fire 3
KDB
NFPA Health 1
KDB
Pc [3710.00; 3710.00]
kPa
Pc 3710.00
kPa
KDB
Pc 3710.00 ± 20.00
kPa
NIST
Pc 3710.00 ± 40.00
kPa
NIST
Inp [596.60; 662.00]
Inp 597.50
NIST
Inp 597.90
NIST
Inp 600.00
NIST
Inp 652.00
NIST
Inp 644.00
NIST
Inp 628.00
NIST
Inp 652.00
NIST
Inp Outlier
NIST
Inp 642.00
NIST
Inp 644.00
NIST
Inp 628.00
NIST
Inp 652.00
NIST
Inp Outlier
NIST
Inp 642.00
NIST
Inp 644.00
NIST
Inp 628.00
NIST
Inp 625.00
NIST
Inp 614.00
NIST
Inp 615.00
NIST
Inp Outlier
NIST
Inp 628.00
NIST
Inp 619.00
NIST
Inp 614.00
NIST
Inp 622.00
NIST
Inp 614.00
NIST
Inp 625.00
NIST
Inp 628.00
NIST
Inp 630.00
NIST
Inp 619.00
NIST
Inp 659.00
NIST
Inp 658.00
NIST
Inp 629.00
NIST
Inp 626.00
NIST
Inp 628.00
NIST
Inp 626.00
NIST
Inp 628.00
NIST
Inp 628.00
NIST
Inp 634.00
NIST
Inp 631.00
NIST
Inp 626.00
NIST
Inp 628.00
NIST
Inp 628.00
NIST
Inp 636.00
NIST
Inp 636.00
NIST
Inp 636.00
NIST
Inp 628.00
NIST
Inp 631.00
NIST
Inp 636.00
NIST
Inp 597.50
NIST
I [966.00; 1048.00]
I 1048.00
NIST
I 1028.00
NIST
I 1026.00
NIST
I 1048.00
NIST
I 1028.00
NIST
I 1026.00
NIST
I 1002.00
NIST
I 1021.00
NIST
I 1012.00
NIST
I 1004.00
NIST
I 1029.00
NIST
I 1000.00
NIST
I 1011.00
NIST
I 1000.00
NIST
I 1000.00
NIST
I 997.00
NIST
I 1003.00
NIST
I 1003.00
NIST
I 975.00
NIST
I 1008.00
NIST
I 987.00
NIST
I 1015.00
NIST
I 1014.00
NIST
I Outlier
NIST
I 1011.00
NIST
I 975.00
NIST
I 1048.00
NIST
I 1026.00
NIST
I 1029.00
NIST
I 987.00
NIST
S°gas 362.80 ± 6.70
J/mol×K
NIST
S°liquid 229.30
J/mol×K
NIST
Tboil [355.35; 376.15]
K
Tboil 375.50
K
KDB
Tboil 375.20
K
NIST
Tboil 375.00
K
NIST
Tboil 375.40
K
NIST
Tboil 375.00 ± 0.50
K
NIST
Tboil 375.40 ± 0.40
K
NIST
Tboil Outlier K
NIST
Tboil 373.15 ± 2.00
K
NIST
Tboil 374.65 ± 1.00
K
NIST
Tboil 375.40 ± 1.00
K
NIST
Tboil 375.05 ± 0.50
K
NIST
Tboil 375.45 ± 0.50
K
NIST
Tboil 374.55 ± 1.00
K
NIST
Tboil 374.85 ± 0.50
K
NIST
Tboil 374.65 ± 1.00
K
NIST
Tboil 375.15 ± 1.00
K
NIST
Tboil 375.40 ± 1.00
K
NIST
Tboil 374.95 ± 0.50
K
NIST
Tboil 375.50 ± 0.30
K
NIST
Tboil 374.65 ± 1.00
K
NIST
Tboil 375.60 ± 0.60
K
NIST
Tboil 375.20 ± 0.50
K
NIST
Tboil 375.15 ± 0.50
K
NIST
Tboil 374.65 ± 1.00
K
NIST
Tboil 373.45 ± 1.50
K
NIST
Tboil 375.50 ± 0.10
K
NIST
Tboil 375.40 ± 0.20
K
NIST
Tboil 375.20 ± 0.50
K
NIST
Tboil Outlier K
NIST
Tboil 375.25 ± 0.50
K
NIST
Tboil 374.75 ± 0.50
K
NIST
Tboil 374.00 ± 3.00
K
NIST
Tboil 374.15 ± 2.00
K
NIST
Tboil 375.65 ± 2.00
K
NIST
Tboil 375.05 ± 0.50
K
NIST
Tboil 375.46 ± 0.20
K
NIST
Tboil 375.65 ± 2.00
K
NIST
Tboil 375.15 ± 2.00
K
NIST
Tboil 375.65 ± 2.00
K
NIST
Tboil 373.90 ± 2.00
K
NIST
Tboil 374.96 ± 0.50
K
NIST
Tboil 374.90 ± 1.00
K
NIST
Tboil 376.15 ± 2.00
K
NIST
Tc [543.70; 545.00]
K
Tc 543.70
K
KDB
Tc 543.70 ± 0.50
K
NIST
Tc 543.70 ± 0.70
K
NIST
Tc 545.00
K
NIST
Tc 544.90
K
NIST
Tfus [262.75; 264.30]
K
Tfus 264.30
K
KDB
Tfus 263.45
K
Aq. Sol...
Tfus 264.20 ± 0.50
K
NIST
Tfus 263.95 ± 0.50
K
NIST
Tfus 262.75 ± 0.60
K
NIST
Ttriple 264.00 ± 0.20
K
NIST
Vc 0.324
m3 /kmol
Joback Calculated Property
Temperature Dependent Properties
Property
Value
Unit
Temperature (K)
Source
Cp,gas [167.70; 222.80]
J/mol×K
[381.35; 576.05]
Cp,gas 167.70 ± 4.10
J/mol×K
381.35
NIST
Cp,gas 168.60 ± 4.10
J/mol×K
384.65
NIST
Cp,gas 169.40 ± 4.10
J/mol×K
387.45
NIST
Cp,gas 171.90 ± 4.10
J/mol×K
396.05
NIST
Cp,gas 172.40 ± 4.10
J/mol×K
398.05
NIST
Cp,gas 180.30 ± 4.10
J/mol×K
425.95
NIST
Cp,gas 194.30 ± 4.10
J/mol×K
475.25
NIST
Cp,gas 207.20 ± 4.10
J/mol×K
520.85
NIST
Cp,gas 222.80 ± 4.10
J/mol×K
576.05
NIST
Cp,liquid [244.14; 248.86]
J/mol×K
[294.40; 298.15]
Cp,liquid 244.14
J/mol×K
294.40
NIST
Cp,liquid 247.15
J/mol×K
298.15
NIST
Cp,liquid 248.86
J/mol×K
298.15
NIST
Cp,liquid 248.86
J/mol×K
298.15
NIST
Cp,liquid 247.30
J/mol×K
298.15
NIST
η [0.0025042; 0.0058667]
Pa×s
[288.15; 313.15]
η 0.0058667
Pa×s
288.15
Excess ...
η 0.0051080
Pa×s
293.15
Densiti...
η 0.0034781
Pa×s
298.15
Excess ...
η 0.0044740
Pa×s
298.15
Densiti...
η 0.0039086
Pa×s
303.15
Densiti...
η 0.0025042
Pa×s
308.15
Excess ...
η 0.0033871
Pa×s
308.15
Densiti...
η 0.0029086
Pa×s
313.15
Densiti...
Δfus H [0.17; 4.46]
kJ/mol
[146.00; 264.00]
Δfus H 1.96
kJ/mol
146.00
NIST
Δfus H 2.00
kJ/mol
192.50
NIST
Δfus H 0.17
kJ/mol
213.00
NIST
Δfus H 2.24
kJ/mol
262.70
NIST
Δfus H 4.46
kJ/mol
264.00
NIST
Δfus H 4.46
kJ/mol
264.00
NIST
Δvap H [39.04; 52.80]
kJ/mol
[313.00; 375.40]
Δvap H 48.40 ± 0.20
kJ/mol
313.00
NIST
Δvap H 49.00
kJ/mol
327.50
NIST
Δvap H 46.40 ± 0.20
kJ/mol
328.00
NIST
Δvap H 48.50
kJ/mol
331.00
NIST
Δvap H 52.80
kJ/mol
336.50
NIST
Δvap H 51.20
kJ/mol
338.00
NIST
Δvap H 47.30
kJ/mol
341.50
NIST
Δvap H 44.20 ± 0.10
kJ/mol
343.00
NIST
Δvap H 45.80
kJ/mol
349.50
NIST
Δvap H 42.00 ± 0.10
kJ/mol
358.00
NIST
Δvap H 40.30 ± 0.10
kJ/mol
368.00
NIST
Δvap H 39.04
kJ/mol
375.40
NIST
ν [0.0000021; 0.0000051]
m2 /s
[293.15; 323.15]
ν 0.0000051
m2 /s
293.15
Density...
ν 0.0000045
m2 /s
298.15
Density...
ν 0.0000039
m2 /s
303.15
Density...
ν 0.0000034
m2 /s
308.15
Density...
ν 0.0000029
m2 /s
313.15
Density...
ν 0.0000025
m2 /s
318.15
Density...
ν 0.0000021
m2 /s
323.15
Density...
Pvap [2.81; 102.61]
kPa
[303.29; 375.53]
Pvap 2.81
kPa
303.29
Vapor-L...
Pvap 2.85
kPa
303.47
Vapor-L...
Pvap 3.16
kPa
304.67
Vapor-L...
Pvap 3.40
kPa
305.75
Vapor-L...
Pvap 3.43
kPa
305.90
Vapor-L...
Pvap 4.08
kPa
308.35
Vapor-L...
Pvap 4.31
kPa
309.12
Vapor-L...
Pvap 4.55
kPa
309.86
Vapor-L...
Pvap 4.80
kPa
310.61
Vapor-L...
Pvap 5.15
kPa
311.64
Vapor-L...
Pvap 5.43
kPa
312.47
Vapor-L...
Pvap 5.69
kPa
313.15
Vapor-L...
Pvap 5.77
kPa
313.38
Vapor-L...
Pvap 6.08
kPa
314.17
Vapor-L...
Pvap 6.59
kPa
315.44
Vapor-L...
Pvap 7.31
kPa
317.09
Vapor-L...
Pvap 7.99
kPa
318.64
Vapor-L...
Pvap 8.36
kPa
319.37
Vapor-L...
Pvap 8.61
kPa
319.86
Vapor-L...
Pvap 8.84
kPa
320.32
Vapor-L...
Pvap 9.36
kPa
321.32
Vapor-L...
Pvap 9.99
kPa
322.44
Vapor-L...
Pvap 10.41
kPa
323.15
Vapor-L...
Pvap 10.41
kPa
323.15
Vapor-L...
Pvap 10.49
kPa
323.27
Vapor-L...
Pvap 11.03
kPa
324.11
Vapor-L...
Pvap 11.51
kPa
324.91
Vapor-L...
Pvap 12.03
kPa
325.71
Vapor-L...
Pvap 12.55
kPa
326.45
Vapor-L...
Pvap 13.05
kPa
327.18
Vapor-L...
Pvap 13.36
kPa
327.62
Vapor-L...
Pvap 13.97
kPa
328.41
Vapor-L...
Pvap 14.65
kPa
329.33
Vapor-L...
Pvap 15.31
kPa
330.15
Vapor-L...
Pvap 15.97
kPa
330.98
Vapor-L...
Pvap 16.67
kPa
331.80
Vapor-L...
Pvap 17.29
kPa
332.46
Vapor-L...
Pvap 17.87
kPa
333.15
Vapor-L...
Pvap 17.87
kPa
333.15
Vapor-L...
Pvap 17.99
kPa
333.26
Vapor-L...
Pvap 18.65
kPa
333.99
Vapor-L...
Pvap 19.32
kPa
334.69
Vapor-L...
Pvap 20.03
kPa
335.45
Vapor-L...
Pvap 19.33
kPa
335.75
Vapor p...
Pvap 19.84
kPa
336.04
Vapor L...
Pvap 20.67
kPa
336.05
Vapor-L...
Pvap 21.31
kPa
336.68
Vapor-L...
Pvap 21.96
kPa
337.31
Vapor-L...
Pvap 22.63
kPa
337.97
Vapor-L...
Pvap 22.87
kPa
338.15
Vapor-L...
Pvap 23.47
kPa
338.69
Vapor-L...
Pvap 22.66
kPa
339.03
Vapor p...
Pvap 23.97
kPa
339.15
Vapor-L...
Pvap 24.67
kPa
339.68
Vapor-L...
Pvap 25.35
kPa
340.31
Vapor-L...
Pvap 26.00
kPa
340.83
Vapor-L...
Pvap 26.67
kPa
341.39
Vapor-L...
Pvap 27.33
kPa
341.90
Vapor-L...
Pvap 26.00
kPa
341.93
Vapor p...
Pvap 27.97
kPa
342.40
Vapor-L...
Pvap 28.67
kPa
342.92
Vapor-L...
Pvap 28.95
kPa
343.15
Vapor-L...
Pvap 29.31
kPa
343.42
Vapor-L...
Pvap 30.01
kPa
343.94
Vapor-L...
Pvap 30.67
kPa
344.47
Vapor-L...
Pvap 29.33
kPa
344.54
Vapor p...
Pvap 29.94
kPa
344.75
Vapor L...
Pvap 31.35
kPa
344.91
Vapor-L...
Pvap 32.07
kPa
345.39
Vapor-L...
Pvap 32.67
kPa
345.78
Vapor-L...
Pvap 33.48
kPa
346.35
Vapor-L...
Pvap 32.66
kPa
346.91
Vapor p...
Pvap 34.67
kPa
347.18
Vapor-L...
Pvap 35.32
kPa
347.57
Vapor-L...
Pvap 36.07
kPa
348.07
Vapor-L...
Pvap 36.71
kPa
348.49
Vapor-L...
Pvap 37.36
kPa
348.88
Vapor-L...
Pvap 36.00
kPa
349.10
Vapor p...
Pvap 38.01
kPa
349.27
Vapor-L...
Pvap 38.64
kPa
349.64
Vapor-L...
Pvap 39.32
kPa
350.08
Vapor-L...
Pvap 39.96
kPa
350.49
Vapor-L...
Pvap 40.64
kPa
350.83
Vapor-L...
Pvap 41.31
kPa
351.20
Vapor-L...
Pvap 39.96
kPa
351.29
Vapor L...
Pvap 40.00
kPa
351.52
Vapor p...
Pvap 42.08
kPa
351.67
Vapor-L...
Pvap 42.65
kPa
351.95
Vapor-L...
Pvap 43.35
kPa
352.30
Vapor-L...
Pvap 44.00
kPa
352.73
Vapor-L...
Pvap 44.65
kPa
353.02
Vapor-L...
Pvap 44.87
kPa
353.15
Vapor-L...
Pvap 45.35
kPa
353.44
Vapor-L...
Pvap 45.97
kPa
353.73
Vapor-L...
Pvap 44.00
kPa
353.75
Vapor p...
Pvap 46.63
kPa
354.10
Vapor-L...
Pvap 47.28
kPa
354.41
Vapor-L...
Pvap 47.95
kPa
354.72
Vapor-L...
Pvap 48.65
kPa
355.11
Vapor-L...
Pvap 49.31
kPa
355.43
Vapor-L...
Pvap 49.95
kPa
355.74
Vapor-L...
Pvap 48.00
kPa
355.83
Vapor p...
Pvap 50.75
kPa
356.09
Vapor-L...
Pvap 51.36
kPa
356.45
Vapor-L...
Pvap 49.95
kPa
356.60
Vapor L...
Pvap 52.00
kPa
356.69
Vapor-L...
Pvap 52.61
kPa
356.99
Vapor-L...
Pvap 53.32
kPa
357.35
Vapor-L...
Pvap 54.03
kPa
357.67
Vapor-L...
Pvap 52.00
kPa
357.78
Vapor p...
Pvap 54.67
kPa
357.96
Vapor-L...
Pvap 55.35
kPa
358.25
Vapor-L...
Pvap 55.95
kPa
358.47
Vapor-L...
Pvap 56.63
kPa
358.85
Vapor-L...
Pvap 57.33
kPa
359.15
Vapor-L...
Pvap 58.00
kPa
359.48
Vapor-L...
Pvap 58.59
kPa
359.68
Vapor-L...
Pvap 56.66
kPa
359.90
Vapor p...
Pvap 59.27
kPa
360.01
Vapor-L...
Pvap 59.95
kPa
360.21
Vapor-L...
Pvap 60.59
kPa
360.53
Vapor-L...
Pvap 61.27
kPa
360.83
Vapor-L...
Pvap 61.99
kPa
361.11
Vapor-L...
Pvap 60.05
kPa
361.18
Vapor L...
Pvap 62.61
kPa
361.40
Vapor-L...
Pvap 63.35
kPa
361.72
Vapor-L...
Pvap 61.33
kPa
361.88
Vapor p...
Pvap 64.04
kPa
362.01
Vapor-L...
Pvap 64.67
kPa
362.24
Vapor-L...
Pvap 65.35
kPa
362.54
Vapor-L...
Pvap 66.00
kPa
362.84
Vapor-L...
Pvap 66.71
kPa
363.07
Vapor-L...
Pvap 67.92
kPa
363.56
Vapor-L...
Pvap 66.66
kPa
364.01
Vapor p...
Pvap 69.21
kPa
364.15
Vapor-L...
Pvap 70.64
kPa
364.62
Vapor-L...
Pvap 71.99
kPa
365.21
Vapor-L...
Pvap 70.74
kPa
365.40
Vapor L...
Pvap 73.37
kPa
365.73
Vapor-L...
Pvap 71.99
kPa
366.01
Vapor p...
Pvap 74.75
kPa
366.16
Vapor-L...
Pvap 76.05
kPa
366.67
Vapor-L...
Pvap 77.28
kPa
367.11
Vapor-L...
Pvap 78.63
kPa
367.53
Vapor-L...
Pvap 77.33
kPa
367.90
Vapor p...
Pvap 79.97
kPa
368.02
Vapor-L...
Pvap 78.27
kPa
368.07
Vapor L...
Pvap 78.09
kPa
368.16
Vapor L...
Pvap 81.28
kPa
368.44
Vapor-L...
Pvap 80.20
kPa
368.75
Vapor L...
Pvap 82.53
kPa
368.83
Vapor-L...
Pvap 83.93
kPa
369.31
Vapor-L...
Pvap 82.66
kPa
369.69
Vapor p...
Pvap 85.28
kPa
369.73
Vapor-L...
Pvap 86.63
kPa
370.15
Vapor-L...
Pvap 87.97
kPa
370.56
Vapor-L...
Pvap 89.39
kPa
370.99
Vapor-L...
Pvap 90.76
kPa
371.39
Vapor-L...
Pvap 91.99
kPa
371.77
Vapor-L...
Pvap 89.33
kPa
371.80
Vapor p...
Pvap 90.23
kPa
371.97
Vapor L...
Pvap 93.31
kPa
372.16
Vapor-L...
Pvap 94.67
kPa
372.56
Vapor-L...
Pvap 95.99
kPa
372.95
Vapor-L...
Pvap 97.39
kPa
373.34
Vapor-L...
Pvap 98.71
kPa
373.73
Vapor-L...
Pvap 95.99
kPa
373.79
Vapor p...
Pvap 98.66
kPa
374.56
Vapor p...
Pvap 100.74
kPa
375.14
Vapor L...
Pvap 101.88
kPa
375.38
Vapor L...
Pvap 102.61
kPa
375.53
Vapor L...
n 0 [1.40160; 1.40250]
[293.15; 298.15]
n 0 1.40160
293.15
Volumet...
n 0 1.40250
298.15
Molar h...
n 0 1.40220
298.15
sotherm...
n 0 1.40219
298.15
Ternary...
n 0 1.40180
298.15
Vapor-L...
n 0 1.40190
298.15
Excess ...
n 0 1.40248
298.15
Vapor L...
ρl [795.16; 826.70]
kg/m3
[273.65; 308.15]
ρl 826.70
kg/m3
273.65
Tempera...
ρl 826.30
kg/m3
274.15
Tempera...
ρl 825.80
kg/m3
274.65
Tempera...
ρl 825.40
kg/m3
275.15
Tempera...
ρl 824.90
kg/m3
275.65
Tempera...
ρl 824.50
kg/m3
276.15
Tempera...
ρl 824.00
kg/m3
276.65
Tempera...
ρl 823.50
kg/m3
277.15
Tempera...
ρl 823.10
kg/m3
277.65
Tempera...
ρl 822.60
kg/m3
278.15
Tempera...
ρl 822.20
kg/m3
278.65
Tempera...
ρl 821.70
kg/m3
279.15
Tempera...
ρl 821.30
kg/m3
279.65
Tempera...
ρl 820.80
kg/m3
280.15
Tempera...
ρl 820.30
kg/m3
280.65
Tempera...
ρl 819.90
kg/m3
281.15
Tempera...
ρl 819.40
kg/m3
281.65
Tempera...
ρl 819.00
kg/m3
282.15
Tempera...
ρl 809.00
kg/m3
293.00
KDB
ρl 804.31
kg/m3
298.15
Excess ...
ρl 804.21
kg/m3
298.15
Measure...
ρl 804.28
kg/m3
298.15
Proposa...
ρl 795.16
kg/m3
308.15
Excess ...
Δfus S [0.78; 16.88]
J/mol×K
[146.00; 264.00]
Δfus S 13.44
J/mol×K
146.00
NIST
Δfus S 0.78
J/mol×K
213.00
NIST
Δfus S 16.88
J/mol×K
264.00
NIST
csound,fluid 1177.79
m/s
298.15
Study o...
γ [0.02; 0.02]
N/m
[293.15; 323.15]
γ 0.02
N/m
293.15
Surface...
γ 0.02
N/m
298.15
Surface...
γ 0.02
N/m
303.15
Surface...
γ 0.02
N/m
308.15
Surface...
γ 0.02
N/m
313.15
Surface...
γ 0.02
N/m
318.15
Surface...
γ 0.02
N/m
323.15
Surface...
Datasets
Viscosity, Pa*s
Fixed
Measured
Temperature, K - Liquid
Pressure, kPa - Liquid
Viscosity, Pa*s - Liquid
303.15
101.33
0.0028
Reference
Correlations
Property
Value
Unit
Temperature (K)
Source
Pvap [0.15; 3758.85]
kPa
[264.35; 545.15]
KDB Vap...
Equation ln(Pvp) = A + B/T + C*ln(T) + D*T^2 Coefficient A 1.65600e+02 Coefficient B -1.19501e+04 Coefficient C -2.20829e+01 Coefficient D 1.24464e-05 Temperature range, min. 264.35 Temperature range, max. 545.15
Pvap 0.15
kPa
264.35
Calculated Property
Pvap 1.87
kPa
295.55
Calculated Property
Pvap 12.35
kPa
326.75
Calculated Property
Pvap 52.10
kPa
357.95
Calculated Property
Pvap 159.88
kPa
389.15
Calculated Property
Pvap 389.54
kPa
420.35
Calculated Property
Pvap 801.71
kPa
451.55
Calculated Property
Pvap 1457.58
kPa
482.75
Calculated Property
Pvap 2419.52
kPa
513.95
Calculated Property
Pvap 3758.85
kPa
545.15
Calculated Property
Similar Compounds
Find more compounds similar to Amylene hydrate .
Mixtures
Find more mixtures with Amylene hydrate .
Sources
KDB Pure (Korean Thermophysical Properties Databank) KDB Vapor Pressure Data Crippen Method Vapor pressure of selected aliphatic alcohols by ebulliometry. Part 2 The influence of cation, anion and temperature on the liquid-liquid equilibrium of some pentanols-water system Isothermal vapor-liquid equilibrium and excess molar enthalpies of the binary mixtures furfural + methyl isobutyl ketone, + 2-butanol and + 2-methyl-2-butanol Measurements and correlation at different temperatures of liquid-liquid equilibria of 2-butanol or 2-methyl-2-butanol + 1,2,3-propanetriol + water ternary systems Henry's law constants and infinite dilution activity coefficients of cis-2-butene, dimethylether, chloroethane, and 1,1-difluoroethane in methanol, 1-propanol, 2-propanol, 1-butanol, 2-butanol, isobutanol, tert-butanol, 1-pentanol, 2-pentanol, 3-pentanol, 2-methyl-1-butanol, 3-methyl-1-butanol, and 2-methyl-2-butanol Molar heat capacities for (2-methyl-2-butanol + heptane) mixtures and cyclopentanol at temperatures from (284 to 353) K Speed of sound, density, and heat capacity for (2-methyl-2-butanol + heptane) at pressures up to 100 MPa and temperatures from (293 to 318) K. Experimental results and theoretical investigations (Liquid + liquid) equilibria in ternary aqueous mixtures of phosphoric acid with organic solvents at T = 298.2 K Excess molar volume, viscosity, and refractive index study for the ternary mixture {2-methyl-2-butanol (1) + tetrahydrofuran (2) + propylamine (3)} at different temperatures. Application of the ERAS-model and Peng Robinson Stryjek Vera equation of state Solubilities of some organic solutes in 1-ethyl-3-methylimidazolium acetate. Chromatographic measurements and predictions from COSMO-RS Solubilities of p-coumaric and caffeic acid in ionic liquids and organic solvents Excess molar enthalpies of the binary systems: (Dibutyl ether + isomers of pentanol) at T = (298.15 and 308.15) K Temperature of maximum density for aqueous mixtures of three pentanol isomers sothermal and Isobaric Vapor-Liquid Equilibrium and Excess Molar Enthalpy of the Binary Mixtures of 2-Methoxy-2-methylpropane + 2-Methyl-2-butanol or + 2-Butanol Solubilities of 3-Chlorophthalic Anhydride and 4-Chlorophthalic Anhydride in Different Pure Solvents Proposal for a Viscous Test Mixture Densities, Viscosities, and Vapor Liquid Equilibrium Data of the Binary Mixture 2-Methyl-2-butanol + 2-Methyl-1-propanol Ternary and Binary LLE Measurements for Solvent (4- Methyl-2-pentanone and 2-Methyl-2-butanol) + Furfural + Water between 298 and 401 K Solubility Measurement and Thermodynamic Modeling of N-(4-Methylphenyl-Z-3-chloro-2-(phenylthio)propenamide in 12 Pure Solvents at Temperatures Ranging from 278.15 to 318.15 K Liquid-Liquid Extraction of Biobased Isobutanol from an Aqueous Solution Measurement and Correlation of Solubility of Marbofloxacin in 12 Pure Solvents from 283.15 to 328.15 K Henry's law constants and infinite dilution activity coefficients of propane, propene, butane, isobutane, 1-butene, isobutene, trans-2-butene, and 1,3-butadiene in 2-methyl-1-butanol, 3-methyl-1-butanol, and 2-methyl-2-butanol Experimental and Predicted Results of Anomeric Equilibrium of Glucose in Alcohols Thermodynamic Properties of Mixtures Containing Ionic Liquids. 8. Activity Coefficients at Infinite Dilution of Hydrocarbons, Alcohols, Esters, and Aldehydes in 1-Hexyl-3-methylimidazolium Bis(trifluoromethylsulfonyl) Imide Using Gas-Liquid Chromatography Density, Viscosity, Excess Molar Volume, and Viscosity Deviation of Three Amyl Alcohols + Ethanol Binary Mixtures from 293.15 to 323.15 K Thermodynamic Properties of Mixtures Containing Ionic Liquids. 9. Activity Coefficients at Infinite Dilution of Hydrocarbons, Alcohols, Esters, and Aldehydes in Trimethyl-butylammonium Bis(trifluoromethylsulfonyl) Imide Using Gas-Liquid Chromatography and Static Method Densities, Viscosities, and Refractive Indices for Binary and Ternary Mixtures of N,N-Dimethylacetamide (1) + 2-Methylbutan-2-ol (2) + Ethyl Acetate (3) at 298.15 K for the Liquid Region and at Ambient Pressure Experimental Study of Thermodynamic Properties of Mixtures Containing Ionic Liquid 1-Ethyl-3-methylimidazolium Ethyl Sulfate Using Gas-Liquid Chromatography and Transpiration Method Heat Capacities in the Solid and in the Liquid Phase of Isomeric Pentanols Vapor-Liquid Equilibria Data for Methanol + 2-Propanol+ 2-Methyl-2-butanol and Constituent Binary Systems at 101.3 kPa Excess Molar Enthalpies of 2-Methyl-2-butanol (1) + 1-Alkanols (C1-C5) (2) at 298.15 K Surface Tensions of Three Amyl Alcohol + Ethanol Binary Mixtures from (293.15 to 323.15) K Volumetric Properties of Highly Nonideal Binary Mixtures Containing Ethanoic Acid and Propanoic Acid with Butan-2-ol, Methyl-2-propanol, and 2-Methyl-2-butanol at Different Temperatures Vapor Liquid Equilibria, Excess Enthalpy, and Excess Volume of Binary Mixtures Containing an Alcohol (1-Butanol, 2-Butanol, or 2-Methyl-2-butanol) and 2-Ethoxy-2-methylbutane Vapor Liquid Equilibrium, Excess Molar Enthalpies, and Excess Molar Volumes of Binary Mixtures Containing Methyl Isobutyl Ketone (MIBK) and 2-Butanol, tert-Pentanol, or 2-Ethyl-1-hexanol Solubility of Flavonoids in Organic Solvents Densities and Viscosities of Binary Liquid Mixtures of 2-Butanone with Branched Alcohols at (293.15 to 313.15) K Study of the Effects of Temperature and Pressure on the Thermodynamic and Acoustic Properties of Pentan-1-ol, 2-Methyl-2-butanol, and Cyclopentanol in the Pressure Range from (0.1 to 100) MPa and Temperature from (293 to 318) K Vapor-Liquid Equilibria in Binary Systems Formed by Cyclohexane with Alcohols Solubilities of Cinnamic Acid Esters in Organic Solvents Vapor-Liquid Equilibria in Binary Systems Formed by n-Hexane with Alcohols Temperature Dependence of Limiting Activity Coefficients, Henry s Law Constants, and Related Infinite Dilution Properties of Branched Pentanols in Water. Measurement, Critical Compilation, Correlation, and Recommended Data Joback Method KDB Aqueous Solubility Prediction Method Estimated Solubility Method McGowan Method NIST Webbook
Note: Cheméo is only indexing the data, follow the source links to retrieve the latest data. The source is also providing more information like the publication year, authors and more.
Take the time to validate and double check the source of the data.
Outlier